In this article Iet’s talk about the most common type of solids: THE METALLIC SOLIDS. These are the ones which we use on daily basis. Just look around you and you’ll notice that most man-made objects are either made of a metals or at least contain 1 metallic component. For example, your watch has metal components, and your cooking pan too. Your car, your computer are mostly made of metallic components. If you have a piece of jewelry made up of pure silver or gold, you have a metallic solid in your hands! So let’s explore a little bit this type of solids.
WHAT IS A METALLIC SOLID?
Metallic solids are exactly what their name suggests: a solid made up of metal atoms only. Think of it like a “metals only” exclusive club. You can only be a member of this club if you are made entirely of metal atoms.
A Metallic solid is a substance in which the particles are linked through a crystal lattice of metallic bonds composed of positive ions (cations) that are attracted to each other in a cloud of delocalized electrons. Within this cloud the electrons can move freely between atoms.
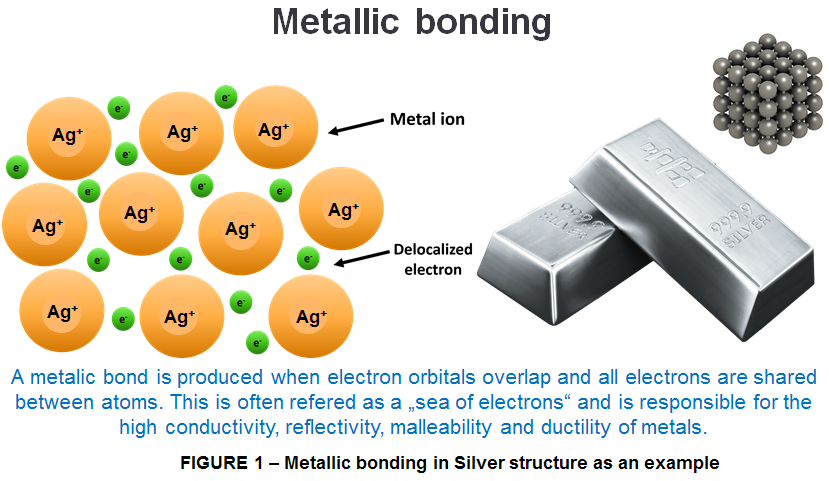
The atoms within such a structure are held together by a unique force known as metallic bonding that gives rise to many useful and varied bulk of properties. As we can see in Fig 1 the metal atoms are held together by metallic bonds and the outer electrons can easily move around within the solid.
When dealing with solids we must always consider that this can be either crystalline (which is a well-organized 3D structure) or amorphous (disordered structure) solids. Metallic solids mostly occurs as crystalline solids, being composed of a large number of small crystals or grains aligned in a variety of different directions. However under a controlled process of temperature (rapidly heated, then rapidly cooled) and pressure, metallic solids in amorphous version can also be obtained. These are often called ”Metallic Glasses”.
We can describe elements as being good or poor metals depending on their metallic character, and the more an element exhibits the properties of metals, then the more metallic that element is. In the periodic table, metallic characters tend to increase from right to left, and down a group. Kind of like electronegativity, if you remember (see the article about Covalent Network Solids).
Metallic bonds are non-directional, meaning that metal atoms can remain bonded while they roll against each other as long as some parts of their surfaces are in contact. These unique properties of metallic bonds are largely responsible for some of the valuable behavior of metals, including their conductivity and malleability. Like for example Gold, Copper, Silver and Aluminium, all having a good malleability and electrical & thermal conductivity.
EXAMPLES OF METALLIC SOLIDS.
From the currently 118 known elements as shown in the Periodic Table (Fig.2), more than 3 quarters of them are metals. And except for mercury (Hg), all metals are solids at room temperature (20°) and atmospheric pressure (1atm).
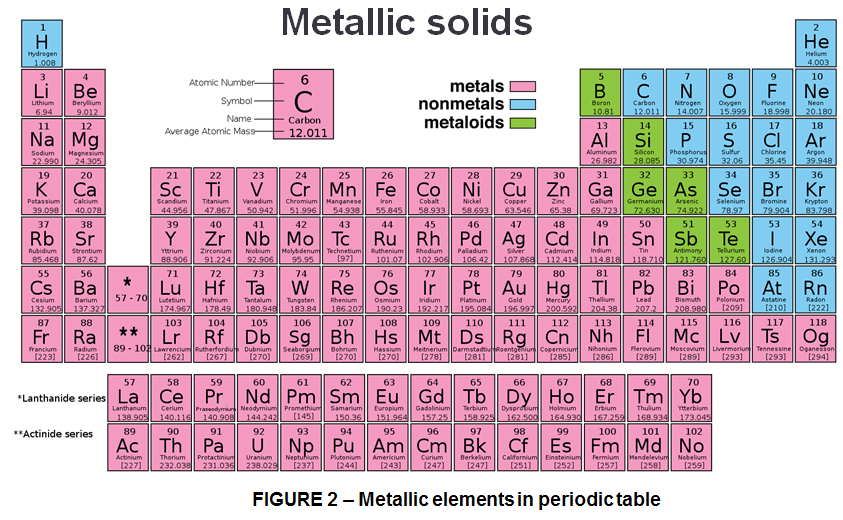
As Periodic table shows we have metallic solids as follows:
- Group I of metals known as the alkali metals (Li, Na, K, Rb, Cs, Fr).
- Group II of metals are called the alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra)
- Groups 3-12 contain many transition metals such as Fe, Cu, Ag, and Au.
- Group 13-16 also have some metals called post-transition metals or poor metals (Al, Ga, In, Tl, Pb, Sn, Bi, and Po).
Note: The elements in Group I in certain conditions can also exhibit characteristics of molecular solids.
PROPERTIES OF METALLIC SOLIDS.
MELTING POINT = as general definition the melting point is the temperature at which a solid turns into a liquid.
This means that when you melt a crystalline solid, its crystal lattice gets broken down and the molecules change into a liquid state. In case of metallic solids there is a large diversity of melting points. Although metallic solids usually have high melting points, some of them, such as the alkali metals (group 1), actually have low melting points.
In the group of transition metals for instance Tungsten ( W) has one of the highest melting points known by chemists. The melting point of a body-centered cubic lattice of Tungsten is around 3400 °C. While another metal, namely mercury (Hg) is already a liquid at -38 °C. Also, you can find basically anything in between among metallic solids. Aluminum (Al) which is a metal found in group 13 (also called III-A in some periodic tables) has a high melting point of 660°C and or Potassium (K) which is a alkali metal (in group 1A) has a melting point of 63.38 °C. Actually all alkali metals melt below 200°C. Several post-transition metals also have low melting points such as Gallium (Ga) at 29.8°C, Indium (In) at 156.6°C or Tin (Sn) at 232°C, whereas most transition metals melt at temperatures above 1000°C. These differences are directly linked to the strength of metallic bonding between the atoms of metal.
ELECTRICAL CONDUCTIVITY: is referred to as the ability to conduct electricity. While you read this article on your computer’ screen, you’re probably not thinking about the wires your computer uses to get the electrical power it needs to run. Those wires are made of metal, most probably copper, simply because generally metals have good electrical conductivity.
Electricity is essentially a flow of electrons from one place to another, and in metallic bonds the outer electrons are relatively free to move between adjacent atoms. This electron mobility means it is easy for an electrical current to move from one end of a piece of metal to the other. Therefore each time when an electron is introduced at one end of a piece of wire by an electric current, this causes other electrons to move from one to another metal atom continuously down the wire, allowing the current to flow. That’s why, metallic solids are good conductors of electricity because of their delocalized electrons which are able to freely move and transmit electrical charges.
THERMAL CONDUCTIVITY: is referred to as the ability to transfer heat, namely how much the atoms or molecules are moving through the internals structure of a substance. Just as metals are good electrical conductors, you probably know from experience that they are good thermal conductors too.
For a solid to conduct heat, the movement of one molecule or atom needs to be easily transferable to its neighbor. Metallic solids do this very well too. The non-directional nature of the metallic bond makes this type of transfer relatively easy, so as result metals conduct heat well. The ability of metallic solids to conduct heat is the reason why most cooking utensils in your kitchen (pots, pans and baking sheets) are made up of some type of metal. That’s because their delocalized electrons can absorb and transfer the heat from the stove or oven faster and then pass it on to the food that’s being cooked!
A very interesting experiment performed by scientists showed that if you add a droplet of a Sodium (Na) and Potassium (K) mixture to water (H2O), then water will turn into a metallic material with a golden color! This happens because it will borrow the outermost electron from both group 1 metals.
HARDNESS: this is a property which measure of how well a material resists deformation when you indent, pierce, hammer, scratch or drop a heavy ball on the same spot, repeatedly. Most metals are relatively hard. Even softer metals, like lead (Pb), gold (Au), or silver (Ag), are still considered hard compared to other materials we handle on a regular basis like wood or rubber.
However among metals the same like melting points, hardness also varies, and there’s something special, and therefore useful, about hardness: it’s a repeatable measurement that says a lot about a part’s other mechanical properties – namely tensile strength and ductility.Hardness is a useful tool in manufacturing environments to quickly qualify material properties because some materials, like carbon and alloyed steels, have a known direct correlation between tensile strength and hardness. And conversely, there is an inverse correlation between hardness and ductility. Because of these consistent relationships, we can closely approximate a metal’s strength by conducting a hardness test. Along with the mechanical specifications from a chosen material grade, we use hardness readings to infer a great deal about a material’s resistance to external forces.
DUCTILITY & MALLEABILITY: Metallic solids are considered malleable and ductile and also many being very hard and strong in the same time. They do not shatter and therefore make themselves useful as great manufacturing materials.
- A malleable metal is a metal that can be battered into thin sheets without breaking it.
- A ductile metal is a metal that can be stretched into different shapes or drawn in long wires without breaking.
These materials exhibit some permanent deformation before they fracture under loading. This type of deformation, in which a material changes shape permanently is known as plastic deformation. Many metals are ductile because the bonds between the atoms allow the atoms to slide over each other. This property is essential in many engineering applications, such as construction, where materials like steel are often used because of their high ductility and ability to withstand external loads and stresses. In this case the relationship between ductility and metallic bonding is a direct one. Metallic bonding is exactly the type of chemical bonding that holds the atoms of metallic elements together, and it is responsible for the unique properties of metals, such as ductility, electrical conductivity, and thermal conductivity.
For instance why copper (Cu) is a great choice of metal to make electrical wiring, is exactly because of the ability of copper to conduct electricity and because copper is ductile and can be molded into the shape of a wire.
As another example, gold (Au) can be hammered into thin gold leaves that are widely used in decorations. And, if that is not interesting enough, you can now even buy gold leaf flakes to use next time you cook your favorite dish! Many malleable materials are also ductile, although the two properties do not occur together; for example lead is highly malleable but has low ductility.
Metallic malleability and ductility are a crucial reason that metals are so useful. Their electrical conductivity would be much less useful if it weren’t possible to stretch them into wires that could then be bent and shaped at room temperature for an incredible array of applications. They also create some drawbacks though. Metal jewelry can be crushed and deformed in the bottom of a purse, or a metal figurine can be dented if it’s dropped. Manufacturers must consider all the properties of the materials they plan to work with to find the best option for each application.
SOLUBILITY: Melting is one way of changing a solid’s shape. Another approach is dissolving the solid into some type of liquid, in this case referred to as a solvent. The extent to which a solid dissolves in a particular solvent is called its solubility. Solids can be dissolved into a variety of types of solvents, but for now we will focus on solubility in water. Dissolving a metal requires breaking metallic bonds, these types of bonds are very strong and hard to break. Therefore, metals are generally not soluble in water.
DENSITY: defined as the amount of mass that exists in a certain volume, is another important property that depends on the solid’s structure and composition. For example, metallic solids do not all share a similar arrangement of atoms. The ions in a metal lattice are closely packed, this giving the metals a High density, as there is little empty space between the atoms. However the atoms and molecules that make up crystals can pack in many different ways, which affects density. Therefore the density of the metal atoms depends upon the mass of the metal ions, their radius and how they are packed together. For instance Lithium, which is the least dense metal at 0.534 g/cm3, is an example of a Low density metal.
LUSTER : Also because of the delocalized electrons in their structure, metallic solids can reflect light, making metals to exhibit a lustrous (shiny) appearance.
METALLIC ALLOYS
Usually we don’t use metals as pure elements. And this is the most interesting part about metallic solids: metals easily combine with each other resulting a solid much useful properties versus each pure element. We call these Metallic Alloys.
For example the melting point of solder (50% lead and 50% tin) is 490 K while that of lead is 600 K and that of tin is 505 K. So as you can see it’s much easier to work with Solder than either individually with Lead (Pb) or Tin (Sn).
An alloy is referred to as a substance that is made up of a mixture of 2 or more elements with metallic properties. Alloys can be either interstitial or substitutional / or a mix of both (Fig. 3).

Interstitial alloys are alloys that form between elements of different radii. These alloys have a more rigid lattice, are less ductile, and are also less malleable compared to metallic solids. A common example of an interstitial alloy is steel, which is made up of carbon and metal ions of Iron+ Cr + Ni.
Substitutional alloys are alloys that form between metals of very similar radii. Because of the similar sizes, some of the metal atoms initially present get replaced by another metal atom. A common example of a substitutional alloy is brass, which is made up of copper (Cu) and zinc (Zn) atoms. In brass, the copper atoms get replaced by zinc atoms. Likewise Bronze which is made up of copper (Cu) and tin (Sn).
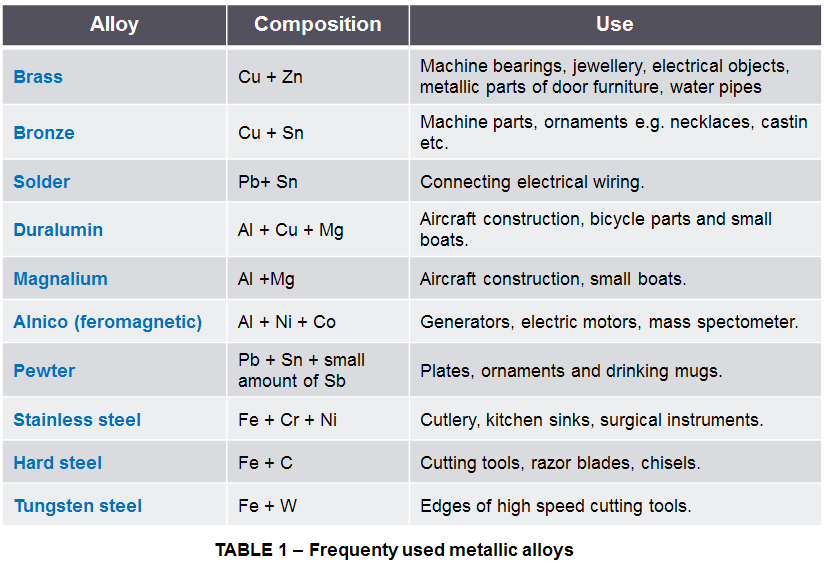
Some of the most common examples of metallic alloys are presented in the Table 1.

Leave a comment