During the wintertime when it snows, if you are like most school kids, you get pretty excited about snowy days. On your day off, you might go outside and play in the snow as you watch the snowflakes fall. When a snowflake lands on your gloved hands, you can see the shape of the snowflake for a brief second before it melts. Snowflakes are all unique in pattern, but they are all hexagonal. Why is this? Well, it’s because snow (ice) is a molecular solid. In this article I’ll tell you more about this type of solid. So let’s dive in.
WHAT IS A MOLECULAR SOLID?
A Molecular solid is = composed of a weak crystalline lattice made of atoms or molecules covalently bonded attracted to each other by weak electrostatic forces (called van der Waals forces). Electrostatic, meaning that they are caused by the attraction/repulsion of electrical charges.
Crystalline lattices are an ordered, repeating arrangement of atoms/molecules and van der Waals forces are weak forces that exist between atom/molecules. These forces also determine how the lattice will be structured. Unlike the stong covalent bonds in CN Solids (CN= Covalent Network), there are 3 main types of weak van der Waals forces, and each of the them correspond to a molecular solid. The main “point” of van der Waals forces is that they are caused by the attraction of a partial negative charge (δ-) to a partial positive one (δ+). How these charges occur is dependent on the type of force that holds the molecules together, which I get into just a bit.
According to the 3 van der Waals forces, the 3 types of molecular solids are:
- NON-POLAR SOLIDS
- POLAR SOLIDS
- HYDROGEN-BONDED SOLIDS
NON-POLAR SOLIDS
For such solid to occur, the bonds they are made of are very much influenced by the electronegativity of the involved atoms.
Electronegativity is the tendency for an atom to pull electron density towards itself. The closer an element is to fluorine on the periodic table (top-right), the more electronegative it is. The values of Electronegativity on Pauli scale are between 0.7 and 4., Flourine (F) having the biggest at 3.98.
When a solid species is non-polar, this happens for one of the next 2 reasons:
- The molecule is symmetrical, canceling out any polarity.
- The difference in electronegativity is less than 0.5.
The non-polar molecules DO NOT HAVE a positive and negative side becasue the electrons are evenly distributed among the atoms making up the molecule. However, even if the molecule is symmetrical, some non-polar molecules may be made up of polar bonds. The symmetrical shape insures an even distribution of the electrons also when the bonds making up the molecule are polar covalent (have unequal sharing). For instance, in carbon dioxide CO2 (when it’s solid becomes dry ice), the oxygen and carbon form polar covalent bonds. However due to symmetry of the molecule the separation of charge cancel out making the molecule non-polar.
In turn the molecular non-polar solids are also held together by London dispersion forces which are are the weakest inter-molecular electrostatic forces between a non-polar species with an instantaneous dipole and a non-polar species with an induced dipole. (as shown in Fig. 1)

The induced dipoles are considered temporary since they will disappear when moved away from a molecule with a dipole. Most molecular solids are non-polar.
POLAR SOLIDS
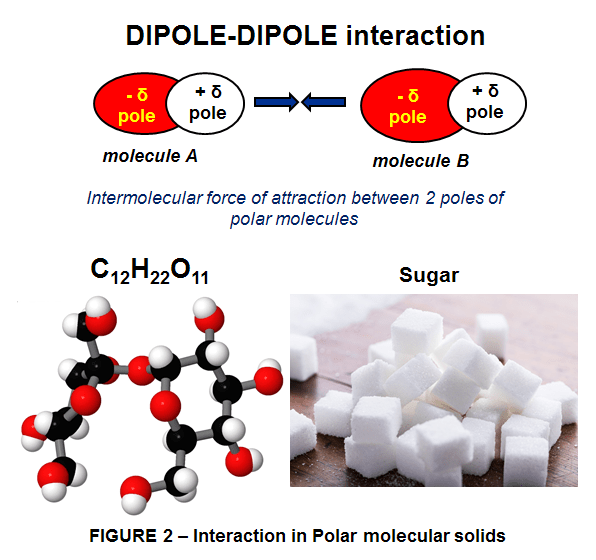
The polar molecules HAVE a positive side and a negative side due to the uneven distribution of electrons in the molecule. Here the molecular solids are held together by dipole-dipole interactions. The dipole-dipole forces are stronger than London forces, making substances with such kind of interaction between the molecules in their structure, to have higher melting points than molecules held by weaker London forces, namely non-polar molecular solids.The bonding electrons in polar molecular solids are pulled closer to the atoms in the molecule with the greatest electronegativity. This makes one end of the molecule slightly negative, while the other end of the molecule is slightly negative, such as in sucrose ( C12H22O11 a.k.a. sugar – Fig 2.)
HYDROGEN-BONDED SOLIDS
They are the stongest intermolecular attractions. Hydrogen (H) bonding is an unusually stong form of dipole-dipole interaction, in other words this results in a stonger Polar Molecular Solid. Substances having molecules held together by Hydrogen bonds tend to have higher boiling points than molecules held together by other intermolecular forces.

When hydrogen is bonded to a very electronegative atom (usually N (nitrogen), O (oxygene) , or F (fluorine), it will have a large, partial positive charge. Because of this, the hydrogen will be attracted to the lone pairs (non-bonded electrons) of a nearby electronegative atom (again N, O, or F). This attraction is referred to as hydrogen bonding and is actually much stronger than in the other 2 types meantioned earlier, because here they have a large difference in electronegativity between H and N, O or F.
EXAMPLES OF MOLECULAR SOLIDS.
Apart from the examples already mentioned (Sugar as polar and Ice as hydrogen-bonded) there are some others very common molecular solids.
NON-POLAR examples: Methane (CH4), all 7 diatomic elements (hydrogen (H2), nitrogen (N2), fluorine (F2), oxygen (O2), iodine (I2), chlorine (Cl2) and bromine (Br2), carbon dioxide CO2 (in solid form know as dry ice). Alkali metals are also considered to have molecular solid caracters.

POLAR examples: sulphur dioxide SO2, hydrochloric acid HCl

HYDROGEN BONDED examples: The molecules of such solids contain polar covalent bonds between H and F, O or N atoms. For instance, water (ice), hydrogen fluoride HF, ammonia NH3, melanin, indigo, dyes, color pigments.

PROPERTIES OF COVALENT NETWORK SOLIDS.
Molecular solids get their properties from the weak van der Waals forces that hold them together. Here are some of the common properties of molecular solids:
SOLUBILITY: by definition dissolving a solid requires breaking different types of bonds for different types of solids. In our case here, dissolving a molecular solid requires breaking only weak intermolecular forces, not the covalent bonds that actually hold the individual molecules together. Therefore, molecular solids are relatively soluble, as you might have been able to guess given how we use sugar in so many drinks. But this also depends on the 3 types of molecular solid involved.
- Non-polar molecular solids: will not dissolve in water, but will dissolve in a nonpolar solvent, such as benzene C6H6 and octane C₈H₁₈.
- Polar molecular solids: dissolve easily in water.
- Hydrogen-bonded molecular solids: are also soluble in water.
HARDNESS: Because of weak van de Waals forces, molecular solids have low density and hardness. For instance the peculiar molecular structure of ice results in its being less dense than liquid water, and it can be argued that without this property life on Earth would have never have come into existence. On a less existential level, it means that we can go ice skating on frozen ponds in the winter even if it hasn’t frozen all the way through.
MELTING POINT: The strengths of the attractive forces between the units present in different crystals vary widely, as indicated by the melting points of the crystals. For molecular solids, melting means breaking the weak intermolecular forces (the forces between different molecules), not the strong covalent bonds that hold the individual molecules together, so a compound like sugar can be easily melted on your stovetop. Most molecular solids melt well below ~300 °C.
Small symmetrical molecules (nonpolar molecules), such as H2, N2, O2, and F2, have weak attractive forces and form molecular solids with very low melting points (below -200 °C). Substances consisting of larger, nonpolar molecules have larger attractive forces and melt at higher temperatures. Molecular solids composed of molecules with permanent dipole moments (polar molecules) melt at still higher temperatures. Examples include ice (melting point, 0 °C) and table sugar (melting point, 185 °C).
Carbon dioxide (CO2) consists of small, nonpolar molecules and forms a molecular solid with a melting point of −78 °C. Iodine (I2) consists of larger, nonpolar molecules and forms a molecular solid that melts at 114 °C.
To have a general overview about the melting points of molecular solids even there are of few exceptions, we can think of it like this,, from the lowest to the highest:
- Lowest: London dispersion forces.: NON-POLAR (below 0°C)(except Iodine)
- Medium Dipole-dipole: POLAR (between 0°C and 100°C)(except sugar)
- Highest: HYDROGEN BONDED. (from 100° C up to 300°C) (except water)
ELECTRICAL CONDUCTIVITY: Molecular solids are nonconductive.To understand why molecular solids are bad electrical conductors, let’s look at what makes a good conductor. Conductors are basically “electron highways”. They allow electrons to flow freely through them. Molecules that are good conductors allow their electrons to move freely. Molecular solids are composed of neutral molecules, so they have no free electrons. This means that electrons cannot flow freely, so they are poor conductors of electricity. But here it also depends of level of impurities in the structure of a molecular solid. For instance pure water is an excellent insulator and does not conduct electricity. The thing is, you won’t find any pure water in nature, so don’t mix electricity and water.
Water stops being an excellent insulator once it starts dissolving substances around it. Salts, such as common table salt (sodium chloride (NaCl)) is the one we know best. In chemical terms, salts are ionic compounds composed of cations (positively charged ions) and anions (negatively charged ions). In solution, these ions essentially cancel each other out so that the solution is electrically neutral (without a net charge). Even a small amount of ions in a water solution makes it able to conduct electricity (so definitely don’t add salt to your “lightning-storm” bathwater). When water contains these ions it will conduct electricity, such as from a lightning bolt or a wire from the wall socket, as the electricity from the source will seek out oppositely-charged ions in the water. Interestingly, if the water contains very large amounts of solutes and ions, then the water becomes such an efficient conductor of electricity that an electrical current may essentially ignore a human body in the water and stick to the better pathway to conduct itself—the masses of ions in the water. That is why the danger of electrocution in sea water is less than it would be in bathwater.
THERMAL CONDUCTIVITY: They are also poor conductors of heat. Heat conductivity is a “passing” of heat energy from one particle to another. Think of it like passing a ball across a line of people. If the people are shoulder-to-shoulder, it doesn’t take much time to pass the ball along, so many balls can be passed along quickly. If the people are standing a few feet apart, it takes a lot more time, so it is much less efficient. Molecular solids have weak van der waals forces holding them together, so they are relatively far apart. This means the passage of heat takes much longer.

Leave a comment