We usually use the terms liquid and fluid interchangeably in general to refer to something that is in a liquid state, such as water. However, there is a stark difference in both terms. In physics, the term fluid refers to any liquid, gas or other material that continuously changes its shape and form under the influence of any force or stress. The shear modulus of fluid is zero, which means such substances cannot resist any force. Although fluid refers to everything that flows generally, including both gas and liquid phases, the definition of fluid often varies depending upon the branch of science.
Free Flow is a characteristic attribute of fluids that describes the continuous and irreversible change in position of one portion of a material relative to another when it is subjected to shear stress. A Liquid is always a fluid, but a fluid is not always a Liquid, it can be a Gas too.
WHAT IS A LIQUID?
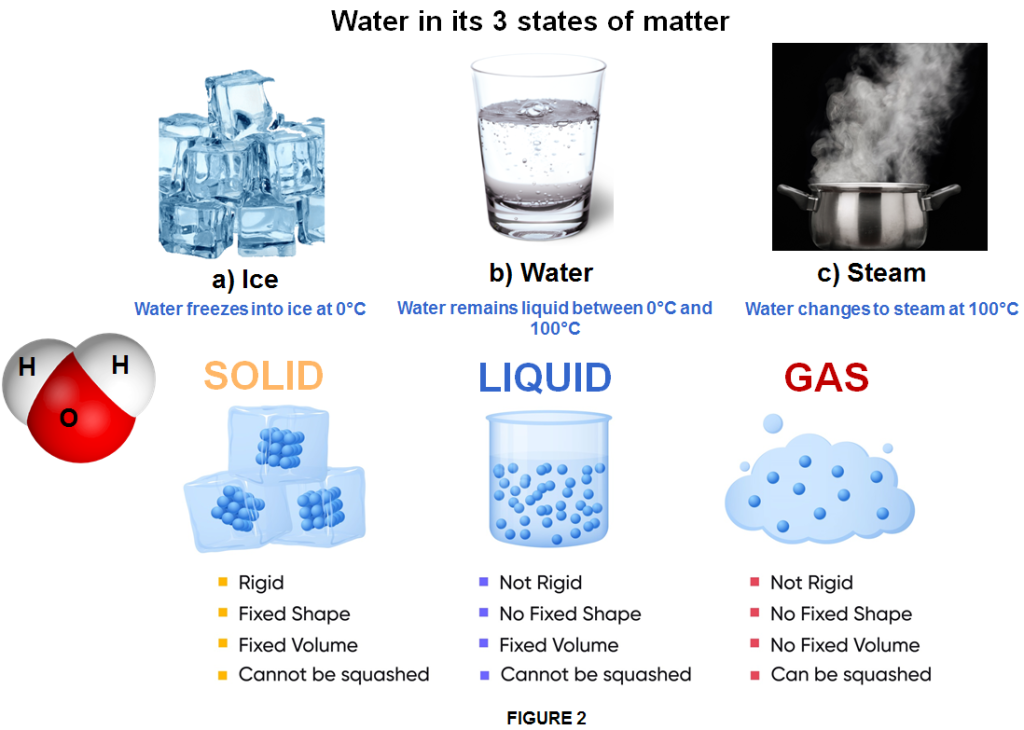
The liquid state of matter is an intermediate phase between solid and gas.
Liquid is: The state of matter in which, the consituent atoms or molecules are closely packed together and the bonds between them are stronger than in gases but weaker than in solids, allowing the particles to move freely.
Because the bonds between particles in a liquid are weak and continuously break and reform as the particles move past each other rather freely, a liquid has no definite shape and takes a shape dictated by its container, except that it does not expand to fill the container (like gases do). However, even if shapeless, a liquid has constant volume.
For example, if a full carton of milk is exactly 1 liter in volume, the milk has the same volume even if you pour it into a 20 L vat. A change in pressure or temperature might alter the volume slightly, but as a whole, the volume remains fairly consistent, unless affected by vaporization or evaporation.
All fluids (liquids and gases) can be broken down into 2 basic types depending on the relationship between viscosity, and shear stress and the rate of the strain.These are: Newtonian, and Non-Newtonian.
NEWTONIAN FLUID = is any fluid (gas or liquid) that exhibits a viscosity that remains constant for a constant temperature, no matter the amount of shear applied (such as mixing or a sudden application of force).
NON-NEWTONIAN FLUID = is a fluid that doesn’t have a constant viscosity and has a variable relationship with shear stress. Therefore the viscosity changes (increases or decreases, depending on the type of fluid involved) due to any external force. In case of liquids, with the change in viscosity, the substance become either more liquid or more solid.

Liquids have FREE FLOW. With this property the atoms or molecules are close together in a limited amount of space, which means that strong intermolecular forces hold liquids together, similar to solids, resulting in a highly dense substance and therefore generally liquids cannot be compressed. Unlike in a solid, the atoms or molecules in liquids are arranged randomly. The density of liquids is higher than that of gases and is typically similar to, or slightly lower than, solids, except in the case of water. Like for any state of matter, the temperature is the main factor which determine the existence of liquid state. Therefore a liquid occurs in a certain range of temperature, outside of which it becomes either a solid or a gas.
When a liquid is heated, the molecular entities gain kinetic energy. If the temperature rises enough, the liquid becomes a gas or reacts with chemicals in the environment. When a liquid is cooled, the molecular entities lose kinetic energy. If the temperature becomes low enough, the liquid becomes a solid.
Water, for example, is the most common liquid on Earth and, in its liquid state, covers a substantial percentage of the surface. However, it is in a liquid state only between the temperatures of 0°C and 100°C. At lower temperatures, it transitions to a solid state and becomes ice (frozen water). At higher temperatures, it transitions to a gaseous state and becomes water vapor (gas). Whether frozen or vapor, the molecular structure of water remains the same as in its liquid state. Every form is still water, just in different states of matter. However, frozen water and water vapor are not liquids, nor are they in a liquid state.
EXAMPLES OF LIQUIDS
Examples of liquids at room temperature (about 20°C) include water, oil, alcohol and mercury. However, liquids can vary substantially from each other depending on their viscosity at a certain temperature. For example, olive oil weighs more than vinegar and is much thicker, causing it to pour more slowly. Olive oil also starts to solidify around 12°C, whereas vinegar starts to freeze at around -2.2°C. Or water becomes gaseous when it is heated gradually, but alcohol can combust when combined with oxygen if heated suddenly and dramatically.


Liquids are often combined to create mixtures. A mixture is either heterogeneous or homogeneous.
A heterogeneous mixture is a combination of substances that do not react chemically with each other. The individual materials are not distributed uniformly, and they retain their individual physical properties, as in the case of oil and vinegar.
A homogeneous mixture, also referred to as a solution, is a type of mixture in which the substances are uniformly distributed and undergo a chemical change. Typically, one substance dissolves into the other to form a new substance with a uniform composition. For example, vodka is a solution made of ethanol and water.
PROPERTIES OF LIQUIDS
Despite the differences between liquids, they can generally be characterized by a set of common physical properties such as:
- SURFACE TENSION/COHESION ;
- VISCOSITY;
- ADHESION;
- EVAPORATION
- VOLATILITY.
Let’s see breifly what all these are, as follows:
SURFACE TENSION – is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet.
This term is typically used only when the liquid surface is in contact with gas (such as the air). If the surface is between 2 liquids (such as water and oil), it is called “interface tension.” When the same kind of particles have the tendency to be attracted to one another we say that the liquid is cohesive. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. The molecule at the surface do not have other like molecules on all sides of them and consequently they cohere more strongly to those directly associated with them on the surface. As result this forms a surface “film” which makes it more difficult to move an object through the surface than to move it when it is completely submersed. This is what holds water together in drops (the shape with the least amount of surface area) or makes it possible to float a pin on its surface. As long as these forces of attraction are undisturbed, they can be surprisingly strong. For example, the surface tension of water is great enough to support the weight of an insect such as a water skipper. When these liquid spheres are distorted by gravity, they form the classic raindrop shape. Water is the most cohesive nonmetallic liquid.
A familiar liquid is mercury metal (Hg). Mercury is an anomaly. It is the only metal we know of that is liquid at room temperature. Mercury also has an ability to stick to itself (surface tension)—a property that all liquids exhibit. Mercury has a relatively high surface tension, which makes it very unique. If we heat liquid mercury to its boiling point of 357°C under the right pressure conditions, we would notice all particles in the liquid state go into the gas state
Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Water at 20°C has a surface tension of 72.8 dynes/cm compared to 22.3 for ethyl alcohol and 465 for mercury. Surface tension is the main characteristic of liquids which is responsible for the physical phenomenon known as Wetting.

Wetting is the degree to which a liquid keeps contact with a solid surface. Whether a liquid wets a surface depends on the strength of the attractive forces within the liquid relative to the forces between the liquid and the surface.
VISCOSITY – is a measure of how easily a liquid flows. A liquid with low viscosity flows easily and is commonly said to be “thin” while a “thick” high-viscosity liquid flows less readily. Viscosity varies according to the molecular size and intermolecular forces in liquid. For example, honey due to its larger molecular structure is more viscous than water. Honey is thicker than water and flows more slowly. Viscosity is determined by bonds between the liquid’s molecules – the stronger the bonds, the more viscous the liquid. Increasing the temperature of a liquid decreases its viscosity, because the molecules have more energy to overcome the intermolecular bonds, allowing the liquid to flow more easily. Viscosity is measured in units called centipoises.

Liquid flow = Liquids with low viscosity, such as water, flow easily, because the bonds between the molecules are weak. In contrast, honey flows much less readily at the same temperature, due to the strength of its intermolecular bonds.
Q: What is the most viscous liquid?
A: Pitch, used for road surfaces, is the most viscous liquid known. It is about 20 billion times more viscous than water at the same temperature.
ADHESION is when forces of attraction exist between different types of particles. Particles of a liquid will not only be attracted to one another, but they are generally attracted to the particles that make up the container holding the liquid, These attractive forces depend of course on the type of liquid and the other substance involved. This explains why water clings to surfaces in different ways, such as glass compared with plastic. Particles of the liquid are drawn up above the surface level of the liquid at the edges where they are in contact with the sides of the container. Adhesion also accounts for capillary action (the tendency for liquid to ascend narrow cylinders or permeable substances) when a liquid is drawn up into a very narrow tube. One example of capillary action is when someone collects a sample of blood by touching a tiny glass tube to the blood droplet on the tip of a pricked finger. The combination of cohesive and adhesive forces means that a slight concave curve, known as the meniscus, exists at the surface of most liquids. The most accurate measurement of the volume of a liquid in a graduated cylinder will be observed by looking at the volume marks closest to the bottom of this meniscus.
EVAPORATION – Because the particles of a liquid are in constant motion, they will collide with one another, and with the sides of the container. Such collisions transfer energy from one particle to another. When enough energy is transferred to a particle at the surface of the liquid, it will eventually overcome the surface tension holding it to the rest of the liquid. Evaporation occurs when surface particles gain enough kinetic energy to escape the system. As the faster particles escape, the remaining particles have lower average kinetic energy, and the temperature of the liquid cools. This phenomenon is known as evaporative cooling.
VOLATILITY can be thought of as how likely a substance will be to vaporize at normal temperatures. Volatility is more often a property of liquids, but some highly volatile solids may sublime at normal room temperature. Sublimation happens when a substance passes directly from solid to gas without passing through the liquid state. When a liquid evaporates inside a closed container, the particles cannot escape the system. Some of the evaporated particles will eventually come into contact with the remaining liquid and lose enough of their energy to condense back into the liquid. When the rate of evaporation and the rate of condensation are the same, there will be no net decrease in the amount of liquid. The pressure exerted by the vapor/liquid equilibrium in the closed container is called the vapor pressure. Increasing the temperature of the closed system will increase the vapor pressure. Substances with high vapor pressures can form a high concentration of gas particles above the liquid in a closed system. This can be a fire hazard if the vapor is flammable. Any small spark, even one occurring from the friction between the gas particles themselves, can be enough to cause a catastrophic fire or even an explosion.

Leave a comment