Glue is an adhesive substance used for sticking objects made of the same or different materials together. Nowadays there are various types of glues used in a large diversity of applications, the most frequently used types are known as PSA ( Pressure-Sensitive Adhesives). Yet the intellectual journey of glue discovery starts with rubber. This belongs to a very important category of glues known as “Rubber-Based Adhesives” (in short RBA). In this article let’s take a closer look at this type of glue: the RBAs.
RUBBER is defined as a material which at room temperature can be stretched to at least twice its original length, and after release of the stress it will return to its approximate original dimensions and shape.
The rubber used in adhesive glue can either be natural or synthetic. Although both can offer comparable properties, there are several differences which should be considered before choosing the best rubber-based adhesive for your application.
NATURAL RUBBER (NR) ADHESIVE
In its natural form Rubber is a sticky tree product from the latex found in various plants and trees, especially it is extracted by tapping the bark of the Pará rubber tree also scientifically known as Hevea brasiliensis, which is a indigenous species of rubberwood native to rainforests in the Amazon region of South America including Brazil, Venezuela, Ecuador, Colombia, Peru, Bolivia and likewise in Central America.

These trees are generally found in low-altitude moist forests, wetlands, riparian zones, forest gaps, and disturbed areas. It is a quick growing tree, often the first to establish itself when a gap in the canopy is produced but may be shaded out as more trees fill in the canopy opening.
The Mesoamerican/Maya cultures made many things with it, including the bouncing balls they used in their ritualistic games. When European explorers reached the American continent in the 16th century, they were amazed by the rubber. They had never seen anything like it before: it has the softness and pliability of leather, but is far more elastic and completely resistant to water. But, despite its obvious value, no one in Europe could find an immediate economic use for it, until the British scientist Joseph Priestley found that it was good for rubbing pencil marks off paper – which is how rubber got its name.
Natural rubber consists of thousands of small isoprene molecules bonded together in a long chain. This molecular trick of linking together units of the same chemical to make a completely different one is common in nature. These types of molecules are called polymers. This term coined in 1833 by Swedish chemist Jöns Jacob Berzelius, comes from Greek – “poly” meaning “many“ and “mer” meaning “unit”. Isoprene is the “mer” in natural rubber. The long polyisoprene chains in rubber are all jumbled up like spaghetti. Due to its long polymer chains, it has great flexibility and becomes elastic when heated.
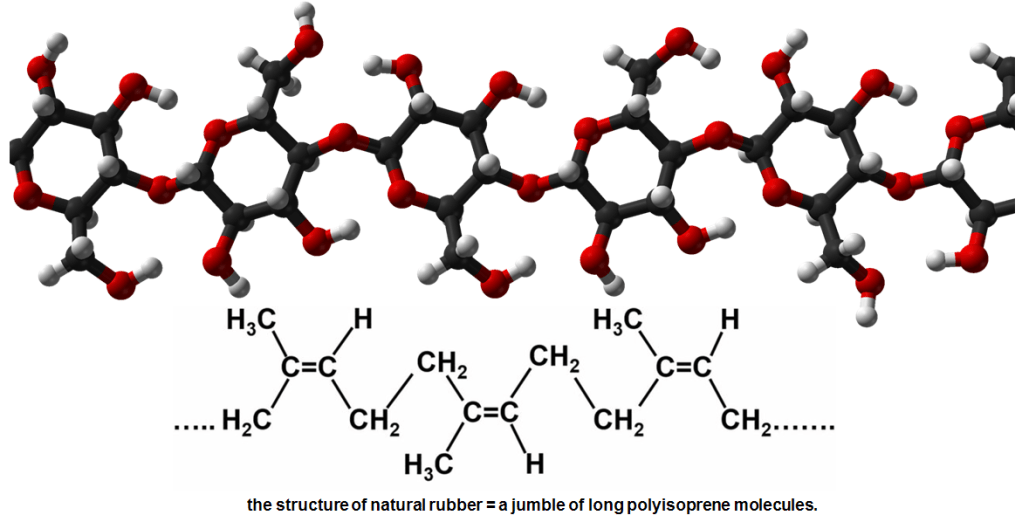
The bonds between each chain are weak, which is why there is not much resistance if you pull the rubber: the chains just unravel. This is what makes rubber so stretchy. It´s rubber`s stretchiness that makes it so sticky. It can mould itself easily and so wedge itself into any space, including the crevices in your hand, which is what makes it grippy. That grip is the reason why rubber is perfect for putting on the handlebars of a bicycle, or for making car tires – it sticks the car to the road strongly enough to create the friction needed to move your wheels forward, but not so strongly that the car gets stuck to the road permanently; similarly it holds your hands to your bicycle´s handlebars firmly enough for them not to slip off accidentally, but you don´t need to worry about getting struck to the bike for ever. These properties make NR adhesives particularly suitable for bonding porous materials such as paper, leather and fabrics, meaning that suitable applications include clothing, footwear, carpet backing and packaging.
On its own, natural rubber does not have any adhesive properties so synthetic additives and resins are added to create formulations that supply the required properties. For example, varying tack and peel adhesion strengths can be achieved to meet the requirements of pressure sensitive applications. NR adhesive formulations often supply the high first tack, short peel dwell times (time taken to reach maximum adhesion), and residue free removability needed for many tape and labeling applications.
Despite its popularity, NR adhesives do have several limitations. When exposed to hot temperatures (+70°C), the adhesive can permanently soften and lose its adhesion and cohesion strength. Natural rubber also rapidly degrades when exposed to UV which breaks down its polymer chains. Together with oxidation, this causes the adhesive to weaken. Therefore, NR adhesive glue is generally not a suitable solution for outdoor applications. Their low chemical, oils and solvent resistance also mean that natural rubber adhesives may not perform well where exposure to these elements is high.
SYNTHETIC RUBBER (SR) ADHESIVE
Synthetic rubbers are manufactured using petrochemical sources in chemical plants, factories and laboratories. Common types include styrene butadiene (SBR), nitrile rubber (NBR), polychloroprene (Neoprene) and silicones. Through the polymerisation of one or more molecular compounds, manufacturers can design and produce products with more complex structures that generally provide greater durability than natural rubbers.
Like natural rubber, synthetic rubbers do not have adhesive properties and additives are needed to create adhesive formulations. However, due to each synthetic rubber having their own beneficial properties, it is easier to develop customized adhesive glue formulations that satisfy unique application and end use requirements. For example, the nitrile rubber-based adhesives (NBR) are known for their high resistance to oils and fuels so are often used for industrial applications such as seals and gaskets. Neoprene based adhesives are characterised by their good resistance to weathering due to their tolerance of moisture, temperature extremes, ozone, UV, and oxidation.
These properties together with their chemical stability, make them suitable for a wide range of outdoor applications. Whilst they might outperform natural rubber adhesives in terms of durability and versatility, synthetic rubber adhesives are generally more costly to manufacture.
FORMULATION OF RBAs
RBAs generally are based in large part on elastomeric polymers. They differ from PSAs in that the adhesive, after application, may or may not be tacky but is expected to hold loads that are semi-structural. By definition with “semi-structural” It means that loads are approximately mid-way between PSAs and structural adhesives. RBAs can be broadly classified into solvent-based systems is generally superior to that of emulsion-based systems, the present day drive to eliminate solvents from the home and workplace has caused rapid development of systems that do not employ these volatile chemicals. RBAs can be further classified into curing and non-curing types.
Since its first use by ancient Maya civilizations, rubber remains an essential polymer for hundreds of everyday products including vehicle tires, footwear, clothing, sports equipment and kitchen appliances. It is also critical to many industrial and commercial applications such as electrical insulation, PPE, seals and gaskets, hydraulics, rubber drive belts and obviously of course adhesives.
RBAs are used especially for furniture made from a laminate of Formica on a less expensive wood base, to bond fabric to foam core in many upholstered furniture applications and are likewise used to a large extent in the shoemaking industry. Many consumers are familiar with the construction mastics used in modern home construction for plywood paneling. RBAs are used to apply floor and wall ceramic tile and are finding increasing use in the bonding of carpet tile to floors. Another basic use of RBAs is in the generation of paper cements. Silicone-based elastomeric cements are often used for glazing of windows but are most often found as sealants.

Leave a comment