Hydrogen is by far much better than any other fuel we use. When burned in combustion the only emission it generates is water vapor so obviosly is the cleanest element 100% Eco-frienly we can ever get. The only problem is how can we get it in a clean way and use it as fuel. To date, there are still many drawbacks which prevent us to completely replace our fossil fuel consumption with hydrogen. But with the progress made in technology in the last 2 decades, there is latelly a growing interest to find multiple solutions in order to overcome as many obstacles as possible. We still have a lot of work to do but for sure sooner than many most of us expect, we will use hydrogen as our main souce of energy. We are just not there yet. Perspectives are very promosing and in this article I want to show you why is hydrogen so much wanted as fuel.
HYDROGEN CONTENT
Hydrogen is the most abundant element in the universe, it’s the element that powers the Sun. The Sun itself is 4 fifth Hydrogen. Yet unfortunately On Earth we don’t find it in important natural sources in its elemental form. On Earth Hydrogen is always locked away in other molecules, while fossil fuels are freely available. So you can’t just dig hydrogen up and burn it. You rarely find it in pure form on Earth because there are lots of other elements around, and hydrogen eagerly grabs on to them with its highly reactive lone electron. That made it difficult for it to compete with fossil fuels. But let’s note that the two weren’t competing on a level playing field. There are hidden costs to coal, oil and gas that were not factored into the equation. Properly accounting for them highlights how important is to free hydrogen from its molecular shackles.
As I said, on Earth we usually find H chemically combined with something else. Much of it is bound up in water (H2O), which covers 75% of the globe’s surface. Likewise, it is present alongside carbon in most organic compounds like food (such as carbohydrates) and fuels made from old organic matter (hydrocarbons, made from hydrogen and carbon in varying quantities).Even in human body, 60% of the atoms in our bodies are hydrogen. Both of these elements (carbon and hydrogen), when they combine with oxygen, generate energy. Carbon generates carbon dioxide (CO2) and the hydrogen only water (H2O). So, the cleaner fuels are those with more hydrogen, as shown in table 1.
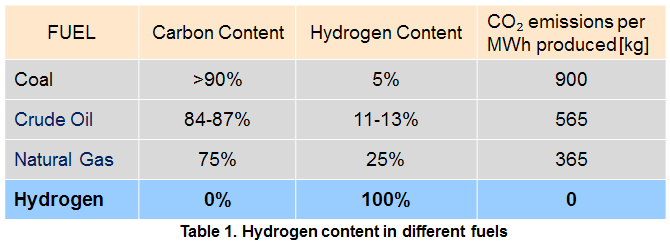
Besides, Hydrogen also likes to bond with itself. When it is generated in a chemical reaction, it generally doesn’t come as a single atom, but as a pair of atoms clinging together: H2. This is the lightest, molecule in existence, and because of that, it moves fast. In the upper reaches of the atmosphere, a hydrogen molecule it is so light that it can quickly reach escape velocity from Earth (11km/s) and shoot off into space, which means there is essentially no hydrogen in our atmosphere either. So getting hold of hydrogen means breaking it free of its bonds with other atoms, which takes at least as much energy as you get back when you burn it. For a while, that was enough to relegate hydrogen to the fringes of the energy market. But recently things started to change and we are now able to make more hydrogen easier and effective. Hydrogen Technology is still at its infant stage, but as soon as we manage to improve things and we’ll control a mature technology then no doubt Hydrogen will replace fossil fuels in most applications.
HYDROGEN POTENTIAL AS ENERGY CARRIER
There is often a topic of debate nowadays, that the amount of energy stored in a substance is directly linked to substance volume and weight. And because hydrogen is the lightest element in existence many people believe that in order to use the same amount of energy contained in 1kg of fossil fuel, you need to store a lot more hydrogen which also occupies more space. For this reason there is still a lot of skepticism about hydrogen abilities to compete with fossil fuels.
The weight and the volume for sure have their pros and cons, but they are not necessarily linked to the amount of energy stored in a substance. In case of hydrogen, the volume is indeed something to take into consideration, while the weight of hydrogen is mostly a positive feature of this energy carrier. Even more so, the low density in terms of weight per volume seems to be confused with low density in terms of the volume of gaseous hydrogen at low pressures. Some of you might say: “Isn’t that the same thing?” The answer is: NO, It’s not the same thing.
To understand this better, first let’s look at what happens at atomic level. From classical studies concerning the structure of matter, we know that most of the mass of an atom is concentrated in its nucleus, which in turn contains protons (carrying a positive electrical charge) and neutrons (free of any electrical charge). This nucleus is orbited by 1 or more electrons (carrying a negative electrical charge). Also a single proton within the nucleus is more than 1800 times more massive than the electron and neutrons have almost the same mass as protons. The radius of the electron’s orbit, which defines the size of the atom, is approximately 100,000 times as large as the radius of the nucleus! Therefore, although atoms of different elements have different configuration of elections and protons, hence different atomic weights, any atom consists largely of empty space. In case of hydrogen in its most frequent occurrence, the H-atom contains 1 electron and 1 proton in its nucleus.

Together, the charges associated with the proton and electron of each hydrogen atom cancel each other out, so that individual hydrogen atoms are electrically neutral. Therefore the reason for the negative answer to the above question is because Hydrogen carries a lot more energy compared to its weight than any other fossil fuel. Now that’s being said, let’s fast forward a bit and analize some practical cases.
CASE 1= Let’s take the liquid hydrogen in order to avoid discussing different pressures at which hydrogen can be stored and compare this with diesel fuel.
In liquid state the density (weight versus volume) of hydrogen is about 72 kg/m³. And here is where confusion starts and why some people are skeptical about the hydrogen potential. The same m³ (cubic meter) holds 835 kg of diesel which is roughly 11.5 times more weight than 1m³ of liquid hydrogen. From that starting point, some argue that a container with liquid hydrogen would carry a lot less hydrogen than a container carrying diesel. In terms of weight yes this is true by a factor of 11.5, but not in terms of energy contents. That’s where the two differ. Per kg hydrogen holds 33 kWh/kg of gross energy. Diesel instead contains 12 kWh/kg of gross energy. So if we do the math, a container carrying the same amount of hydrogen in terms of weight carries 2.75 times the amount of energy compared to a container carrying the same weight of diesel, compared by energy content. This is why some argue that you have to carry a lot more hydrogen in order to move the same amount of energy. But again this is not true, not in terms of weight. The misunderstanding is based on the idea that the density of hydrogen in terms of weight per volume is so much lower than most of the substances we are familiar with, which is indeed true. Yet the confusion is emphasized by the notion (albeit correct) that hydrogen has a very low density (weight versus volume) in its gaseous form, certainly at ambient pressure. Hence hydrogen actually carries a lot of energy compared to its weight. Admittedly, the loading volume of a container carrying the same amount of gross energy in hydrogen, will be larger by about 4.2 times.
CASE 2= Water versus Liquefied Natural Gas (LNG).The density of LNG is a little trickier because it varies with the temperature much more than water does. References give a range density for LNG from 410 to 500 kg/m3.Now let’s see what pops out when assume 410 kg/m3. LNG is mostly methane- CH4, (1 carbon atom linked to 4 hydrogen atoms) , hence the molecular weight =1*12 + 4*1 = 16. So Hydrogen comprises 25% of the mass of any volume of methane. This means 0.25 x 410 kg = 102.5 kg of Hydrogen in a cubic meter of LNG, at a minimum, alternatively, 0.25 x 500 = 125 kg of Hydrogen at maximum.
Water is H2O (2 atoms of hydrogen bonded with 1 atom of oxygen), and has a density of 1000kg/m3 with a molecular mass 2*1 + 16 *1 = 18, hence Hydrogen comprises 11.11% of the mass of any volume of water. This means 0.1111×1000= 111,1kg of Hydrogen in 1m3 of water. So as we can see, the amount of Hydrogen per cubic meter is roughly the same for LNG as for water. Now the efficiency of deriving the Hydrogen from the LNG vs. from water is a different story.
======================================================
Even as a liquid, hydrogen is not very dense. Liquid hydrogen for instance contains only 71kg of hydrogen/m3. Thus, water packs more mass of hydrogen per unit volume, because of its tight molecular structure, than hydrogen itself. This is also true of most other liquid hydrogen-containing compounds as well; a cubic meter of methanol (CH4O) contains 100kg of hydrogen and a cubic meter of heptane (C₇H₁₆) contains 113 kg.
Hydrocarbons are compact hydrogen carriers with the added advantage of having higher energy density than pure hydrogen. When used as vehicle fuel, the low density of hydrogen necessitates that a large volume of hydrogen to be carried to provide an adequate driving range. Generating 1kg of hydrogen through electrolysis uses 9kg of water. For context, supplying hydrogen for a 288-megawatt power plant using 100% hydrogen would require the equivalent of an Olympic-size swimming pool of water every 12 hours. The power plant would need additional water for cooling, which would increase the total water usage to 15 to 20 kg of water per kg of hydrogen. While this is a large amount of water, and could be problematic for water-scarce areas, hydrogen’s water requirements are much less than the amount of water required for the extraction and processing of fossil fuels today. This evidence show us clear enough, that is much better to use hydrogen instead of fossil fuels. And hydrogen is a highly efficient fuel.

good read
Regard Mel
Over 50 Delicious Keto Recipes – http://www.ketodietrecipes.co.uk
LikeLike