Gases are all around us but most of the time we don’t give them much thought. However, along with solids and liquids, gases are one of the main states of matter and the way they behave is vital for life on Earth. For example, when we breathe in, we increase the volume of our lungs, which reduces the pressure inside and causes air to rush in. In this article let’s have a closer look at what is the 3rd state of matter commonly known as GAS.
WHAT IS A GAS?
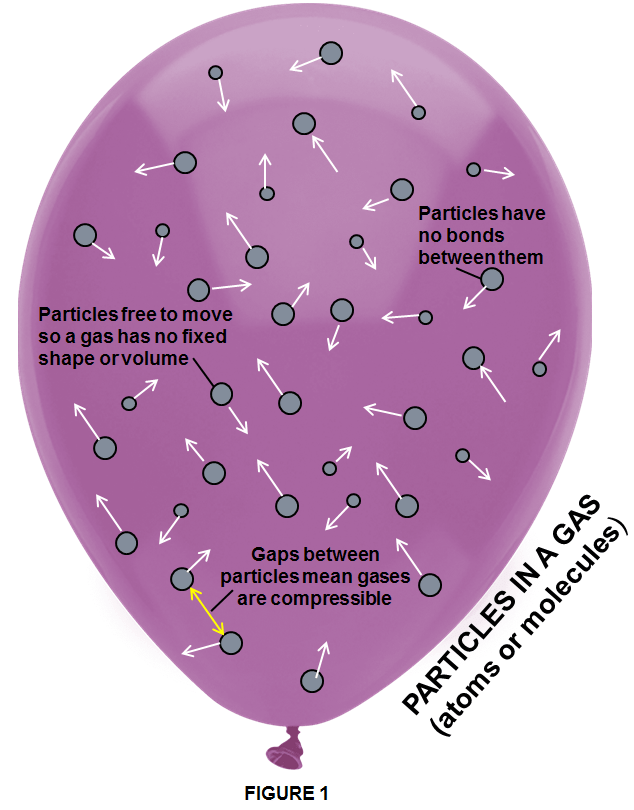
“Gas is a state of matter that has no fixed shape and no fixed volume”.
Gases can be composed of individual atoms or of molecules of two or more atoms which aren’t particularly attracted to one another being in a constant state of motion. These particles are very energetic, they move freely and rapidly around each other, continuously collide with each other, causing them to diffuse, or spread out until they are evenly distributed throughout the volume of their container taking its shape. The gas expands until it is uniformly distributed throughout the container, even in the presence of gravity. If not confined to a container, a gas disperses into space.
This definition suggests, in the first place, that a given mass of gas can occupy any volume whatsoever. Let’s imagine a cylindrical tank filled with a small amount of hydrogen gas (H2) placed in a chemistry laboratory:
- If the top is removed from that tank in a room, the gas escapes from the tank to fill the room.
- If the door to the room is opened, the gas then escapes to fill the building.
- If the building door is also opened, the gas escapes into the outside environment and, at least in theory, then expands throughout the universe.
Neither solids nor liquids, the other two common forms of matter, display this property.
1,700 km/h is THE SPEED AT WHICH OXYGEN MOLECULES MOVE AROUND AT ROOM TEMPERATURE
Likewise, gases take the shape of the container in which they are placed. Suppose the valve of the tank of hydrogen gas is fitted with a rubber hose that leads to a cubic box. When the valve is opened, the hydrogen gas fills the cubic box. Its shape changes from cylindrical to cubic. Liquids also take the shape of their container, although solids do not. The atoms or molecules in a gas are packed more loosely than they are if the substance is in the solid or liquid state. As consequence there is plenty of space between the particles, so gases can be compressed.
EXAMPLE OF GASES
As mentioned earlier in the definition, a gas can be either made of atoms or molecules of the same element such as:
- oxygen (O2) at room temperature (at approximately 20° C)
- hydrogen (H2) at room temperature
- nitrogen (N2) at room temperature
or as a molecule of at least 2 atoms of different elements such as:
- water (H2O) when it exceeds 100 °C at standard atmospheric pressure (sea level)
- carbon dioxide (CO2) at room temperature
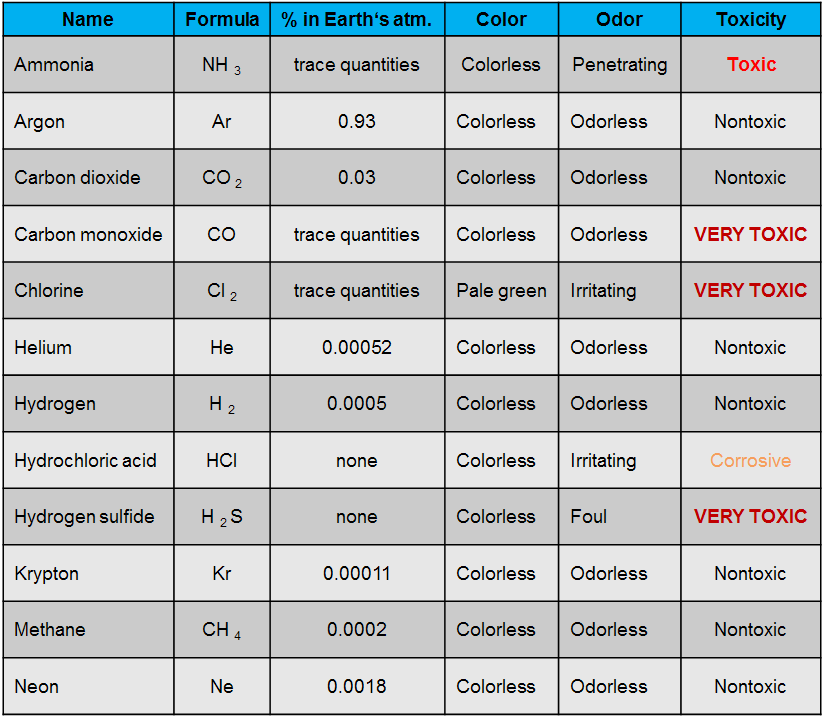
Some of the most frequently used gases are shown in table 1.

When temperatures become sufficiently high, gases such as hydrogen can rapidly combine with gases such as oxygen (O2) or chlorine (Cl2), causing them to combust. Some chemical reactions between gases and other substances occur more slowly. For example, the gradual oxidation of iron results in iron oxide (rust)(Fe2O3). In this case, oxygen (O), a gas, combines with iron (Fe), a solid, to create iron oxide, another solid. Most gases transition to the liquid or solid state if their temperatures drop low enough. For example, if nitrogen (N2) is chilled to a temperature below -196 °C, it liquefies. Liquid nitrogen is used in medicine to destroy minor skin lesions, such as warts, keloids or keratoses. Another gas, carbon dioxide (CO2) , skips the liquid phase and becomes a solid known as dry ice when cooled to below -78 °C.
CHARACTERISTICS OF GASES.
In general, gases tend to be colorless and odorless, although some important exceptions exist.(as for instance those indicated in table 1). Also, most gases are transparent: that is, it is possible to see objects through them rather clearly.
WHY WE CAN’T SEE AIR? => Something is visible only if it affects light, by reflecting it, for example. Air affects light only slightly, so is usually invisible. But large amounts of air scatter blue light noticeably, which is why the sky looks blue.
Gases are characterized by 4 main physical properties. These are:
- Density (ρ) (in kg/m3) – The amount of mass in a specific volume, i.e., how tightly the particles are packed together. Gases have a lower density than solids and liquids. Often in work involving gas the amount of gas is noted as n and measured in moles.
- Volume (V) [in m3 , mL or L(Liters)] – The amount of space the gas occupies.
- Temperature (T) (°C or K) – The absolute temperature of the gas. In work involving gas temperature, the Kelvin scale is often used (0°C = 273.15 K).
- Pressure (P) (atm , mm Hg or Pa) – The amount of force the gas exerts on the container holding it.
All these 4 properties play a vital role in how a gas behaves under different circumstances. Generally the way gases behave is described by a set of 4 gas laws. These laws relate the amount of gas (n), the gas’s , pressure (P), volume (V) and temperature (T) and show how each measure changes when the others change.
The proportional factor that relates the average relative thermal energy of particle in a gas with the thermodynamic temperature of the gas is defined by the Boltzman constant (kB). This is a fundamental constant of physics occurring in nearly every statistical formulation of both quantum and classical physics. It has the exact value of:
kB = 1.380649 × 10-23 m2 kg s-2 K-1
More in detail about gas laws, I will explain in another post. For now I just mention the 4 laws as they are known. These laws are:
- THE AVOGADRO’S LAW (of variable volume & amount of gas) (V & n);
- THE CHARLES’S LAW (of variable volume & temperature)(V & T);
- THE GAY-LUSSAC’S LAW (of variable pressure & temperature)(P & T);
- THE BOYLE’S LAW (of variable pressure & volume)(P & V).
According to these laws gases behave as follows:
- If a gas is in a sealed container and the container’s volume is reduced, the compression heats the gas;
- If a gas is in a sealed container and the container’s volume is increased, the decompression cools the gas;
- When a gas is heated, the atoms or molecules gain kinetic energy and move more rapidly;
- When a gas is cooled, the atoms or molecules lose kinetic energy and move more slowly;
- If a gas is in a sealed container and heated, the pressure increases;
- If a gas is in a sealed container and cooled, the pressure drops.

Leave a comment