We usually use the terms liquid and fluid interchangeably in general to refer to a substance which is in a liquid state, such as water. However, there is a stark difference in both terms. In physics, the term fluid refers to any liquid, gas or other material that continuously changes its shape and form under the influence of any force or stress. The shear modulus of fluid is zero, which means such substances cannot resist any force. Although fluid refers to everything that generally flows, including both gas and liquid phases, the definition of fluid often varies depending upon the branch of science. For example sand – which has a crystalline structure with properties of a solid, it behaves like a fluid too.
Free Flow is a characteristic attribute of fluids that describes the continuous and irreversible change in position of one portion of a material relative to another when it is subjected to shear stress. A Liquid is always a fluid, but a fluid is not always a Liquid, it can be a Gas too, or sand.
WHAT IS A LIQUID?
The liquid state is usually described simply as an intermediate state of matter that occurs between solid and gaseous states, therefore a liquid is:
The state of matter in which, the constituent atoms or molecules from the structure of substance, are closely packed together with the bonds between them which are stronger than in gases, but weaker than in solids, allowing the particles to move freely.
So what gives liquids their ability to flow is exactly their internal structure – they are an intermediate state between the chaos of gas and the static prison (for molecules) of solids. Because the bonds between particles in a liquid are weak and continuously break and reform as the particles move past each other rather freely, a liquid has no definite shape and takes a shape dictated by its container, except that it does not expand to fill the container (like gases do). However, even if shapeless, a liquid has constant volume.
For simple molecules this distinction is unambiguous, no doubt. In practical terms it goes like this: In gases, molecules have enough heat energy to break away from each other and move autonomously. This makes gases dynamic – that’s why they expand to fill the available space – but they have almost no structure.On the other hand, in solids the force of attraction between the atoms and molecules is much greater than the heat energy they possess, causing them to bond together. Thus solids have a lot of structure but little autonomy – when you pick up a bowl, all the atoms of that bowl come together as one object. . Therefore Liquids are indeed an intermediate state between the two. in liquids, The atoms have enough heat energy to break some of the bonds with their neighbors, but not enough to break all of them and become a gas.So they are stuck in the liquid, but able to move around within it. This is what a liquid is – a form of matter in which molecules swim around, making and breaking connections to each other.
For example, if a full carton of milk is exactly 1 liter in volume, the milk has the same volume even if you pour it into a 20 L vat. A change in pressure or temperature might alter the volume slightly, but as a whole, the volume remains fairly consistent, unless affected by vaporization or evaporation.
However, clear distinction between the liquid, gaseous, and solid states holds only for those substances whose molecules are composed of a small number of atoms. For instance when the number exceeds about 20, the liquid may often be cooled below the true melting point to form a glass, which has many of the mechanical properties of a solid but lacks crystalline order. Actually this is a typical structure for an amorphous solid. If the number of atoms in the molecule exceeds about 100–200, the classification into solid, liquid, and gas ceases to be useful. At low temperatures such substances are usually glasses or amorphous solids, and their rigidity falls with increasing temperature—i.e., they do not have fixed melting points; some may, however, form true liquids. With these large molecules, the gaseous state is not attainable, because they decompose chemically before the temperature is high enough for the liquid to evaporate. Synthetic and natural high polymers (e.g., nylon and rubber) behave in this way.
If the molecules are large, rigid, and either roughly planar or linear, as in cholesteryl acetate or p-azoxyanisole, the solid may melt to an anisotropic liquid (i.e., one that has not uniform properties in all directions) in which the molecules are free to move about, but have great difficulty in rotating. . Such a state is called a liquid crystal, and the anisotropy produces changes of the refractive index (a measure of the change in direction of light when it passes from one medium into another) with the direction of the incident light and hence leads to unusual optical effects. Liquid crystals have found widespread applications in temperature-sensing devices and in displays for watches and calculators. However, no inorganic compounds and only about 5% of the known organic compounds form liquid crystals. The theory of normal liquids is, therefore, predominantly the theory of the behavior of substances consisting of simple molecules.
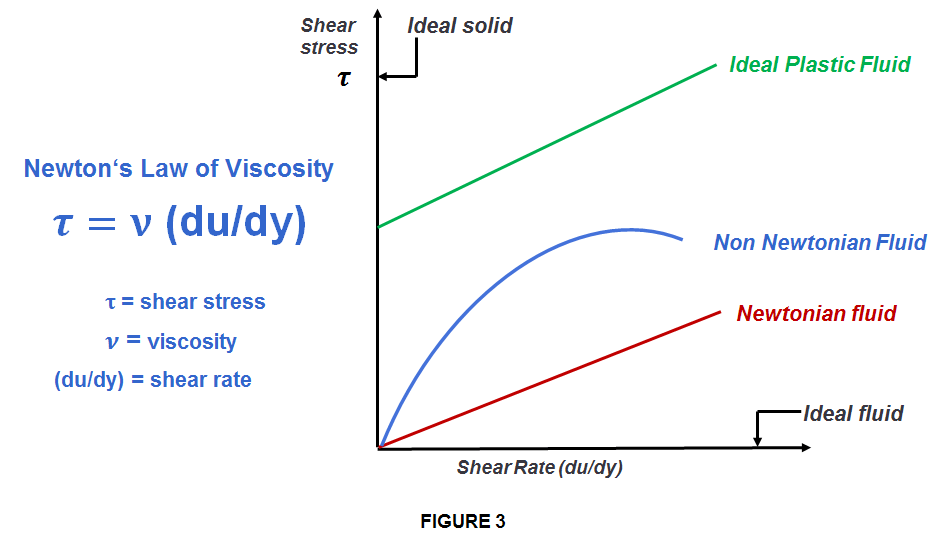
Fluids (liquids and gases) can be broken down into 2 basic types depending on the relationship between viscosity, and shear stress and the rate of the strain. These are: Newtonian, and Non-Newtonian.
NEWTONIAN FLUID = is any fluid (gas or liquid) that exhibits a viscosity that remains constant for a constant temperature, no matter the amount of shear applied (such as mixing or a sudden application of force).

NON-NEWTONIAN FLUID = is a fluid that doesn’t have a constant viscosity and has a variable relationship with shear stress. Therefore the viscosity changes (increases or decreases, depending on the type of fluid involved) due to any external force. In case of liquids, with the change in viscosity, the substance become either more liquid or more solid.

Liquids have FREE FLOW. With this property the atoms or molecules are close together in a limited amount of space, which means that strong intermolecular forces hold liquids together, similar to solids, resulting in a highly dense substance and therefore generally liquids cannot be compressed. But that’s only valid for Newtonian Fluids.
In case of Non-Newtonian, things are a little bit different. As the Non-Newtonian liquids exhibit variable viscosity depending on the external force applied, this may also affect their molecular arrangement in internal structure, hence they might be well compressed.
Unlike in a solid, the atoms or molecules in liquids are arranged randomly. The density of liquids is higher than that of gases and is typically similar to, or slightly lower than, solids, except in the case of water. Like for any state of matter, the temperature is the main factor which determine the existence of liquid state. Therefore a liquid occurs in a certain range of temperature, outside of which it becomes either a solid or a gas.
When a liquid is heated, the molecular entities gain kinetic energy. If the temperature rises enough, the liquid becomes a gas or reacts with chemicals in the environment. When a liquid is cooled, the molecular entities lose kinetic energy. If the temperature becomes low enough, the liquid becomes a solid. Liquids may be further divided into 2 main categories:
- Pure Liquids
- Liquid mixtures.
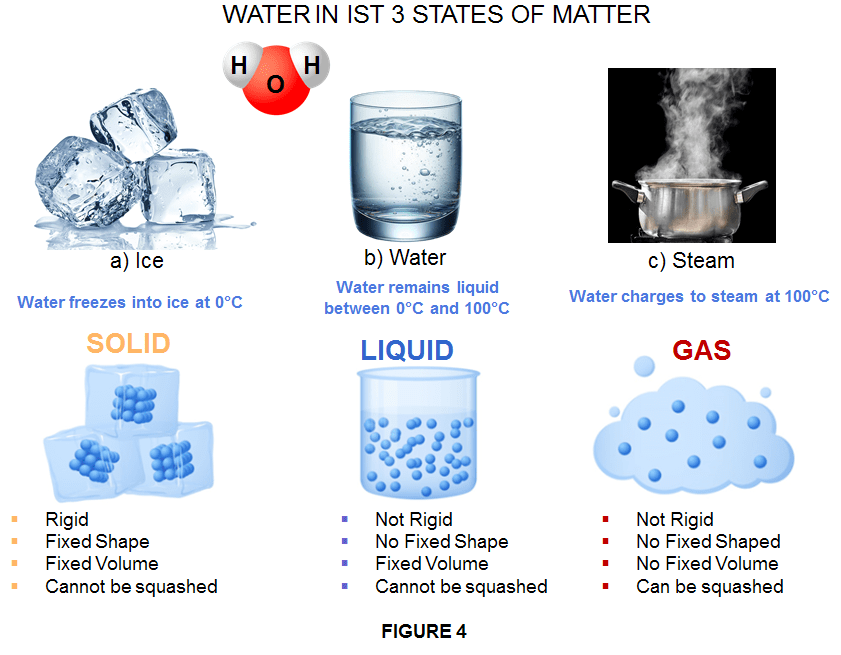
PURE LIQUIDS – Pure substances that are liquid under normal conditions include water, ethanol and many other organic solvents. Water, for example in its pure form is made of simple molecules consisting of 2 atoms of Hydrogen and 1 atom of oxygen, with chemical formula: H2O. It is the most common liquid on Earth and, in its liquid state, covers a substantial percentage of the surface. However, it is in a liquid state only between the temperatures of 0°C and 100°C. At lower temperatures, it transitions to a solid state and becomes ice (frozen water). At higher temperatures, it transitions to a gaseous state and becomes water vapor (gas). Whether frozen or vapor, the molecular structure of water remains the same as in its liquid state. Every form is still water, just in different states of matter. However, frozen water and water vapor are not liquids, nor are they in a liquid state.
On Earth, water is the most abundant liquid, although much of the water with which organisms come into contact is not in pure form but is a mixture in which various substances are dissolved. Such mixtures include those fluids essential to life — blood, for example — beverages, and seawater. Seawater is a liquid mixture in which a variety of salts have been dissolved in water. Even though in pure form these salts are solids, in oceans they are part of the liquid phase. Thus, liquid mixtures contain substances that in their pure form may themselves be liquids, solids, or even gases.
LIQUID MIXTURES – A mixture of liquids is either heterogeneous or homogeneous.
A heterogeneous mixture – is a combination of substances that do not react chemically with each other. The individual materials are not distributed uniformly, and they retain their individual physical properties, as in the case of oil and vinegar.
A homogeneous mixture, also referred to as a solution, is a type of mixture in which the substances are uniformly distributed and undergo a chemical change. Typically, one substance dissolves into the other to form a new substance with a uniform composition. For example, vodka is a solution made of ethanol and water.
THE MAIN CHARACTERISTICS OF LIQUIDS
Examples of liquids at room temperature (about 20°C) include water, oil, alcohol and mercury. However, liquids can vary substantially from each other depending on their viscosity at a certain temperature. For example, olive oil weighs more than vinegar and is much thicker, causing it to pour more slowly. Olive oil also starts to solidify around 12°C, whereas vinegar starts to freeze at around -2.2°C. Or water becomes gaseous when it is heated gradually, but alcohol can combust when combined with oxygen if heated suddenly and dramatically.
The combination of high density and of partial order in liquids has led to difficulties in developing quantitatively acceptable theories of liquids. Understanding of the liquid state, as of all states of matter, came with the kinetic molecular theory, which stated that matter consisted of particles in constant motion and that this motion was the manifestation of thermal energy. The greater the thermal energy of the particle, the faster it moved.

Leave a comment