The category of solids you’ll read about next has very much to do with our food, simply because eat them. These are THE IONIC SOLIDS.
Let just start with an example. Have you ever used Epsom salt to help with muscle soreness and stress? If you do, then let me tell you that Epsom salt is a type of crystalline solid, and more specifically, it is considered an ionic solid. Epsom salt is made up of magnesium and sulfate ions that join together to form magnesium sulfate (MgSO4), and it is believed that Epsom salt has many benefits, from helping with muscle pain to relieving anxiety!
WHAT IS A IONIC SOLID?
By definition of the word “ionic”, indicated that we are dealing with something that is obviously related to, or involves ions.
A ION is a particle, an atom or a molecule with an imbalance of electrical charge.
Therefore ions are charged particles and contain different numbers of protons (+) and electrons (-). Ions form when atoms move into a more stable electron configuration. In technical documentation Ions are identified by a superscript that shows the sign and size of the electric charge – for example Ca+2.
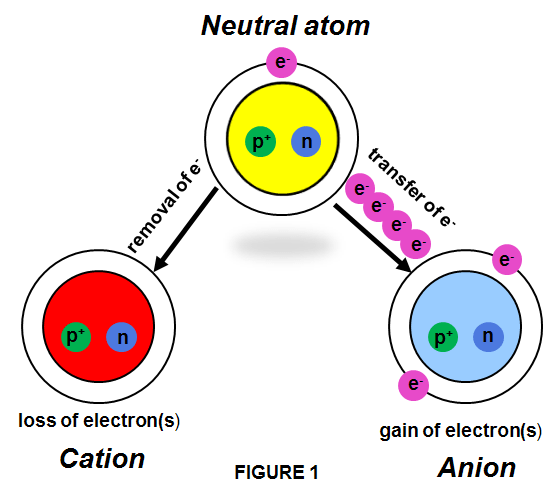
There are 2 types of ions: cations and anions.
A cation is an ion that has lost one or more electrons giving a net positive charge (+), which means it has more protons than electrons. Because one or more electrons are removed to form a cation, the cation of an atom is smaller than the neutral atom. Some examples of cations include the following: Sodium = Na+ Calcium = Ca2+ ; Potassium = K+; Silver = Ag+; Aluminium = Al3+; Hydronium ion = H3O+; Ammonium ion = NH4+; Mercurous ion = Hg2+2; Ferrous Ion = Fe+2, Ferric Ion = Fe +3 .
An anion is an ion that has gained one or more electrons, giving a net negative charge (-), which means it has more electrons than protons. Because electrons are added to form an anion, the anion of an atom is bigger than the neutral atom. Some examples of anions include the following: Chlorine = Cl– ; Hydroxide = OH– , Iodide = I– ; Dichromate = Cr2O7-2 ; Oxide anion = O-2 ; Sulfate anion = SO4-2
There are ionic bonds, ionic compounds, ionization – all of these concepts involve ions.
A Ionic Solid is = a chemical compound which consist of ions joined together in electrostatic interaction by ionic bonds, a type of chemical bond that occurs between a cation (a positively charged ion) and an anion (a negatively charged ion).
A ionic bond, is a chemical bond between atoms where electrons are (mostly) transferred from one atom to another. I say mostly, because there is always some sharing of electrons between atoms, but in Ionic bonds, the sharing is very unequal. The less equal the sharing of the electrons, the more ionic character the bond has. Ionic bonds occur between a metal (usually as cation which is smaller) and a non-metal (usually as anion, which is bigger).
Ionic solids are similar to covalent network solids in one way: There are no distinct molecules. Unlike covalent bonds, ionic bonds transfer their valence electrons between atoms and in ionic bonding, the electronegativity difference between non-metals and metals exceeds 1.7. on Pauling Scale, while in covalent bonds this difference is less than 1.7.
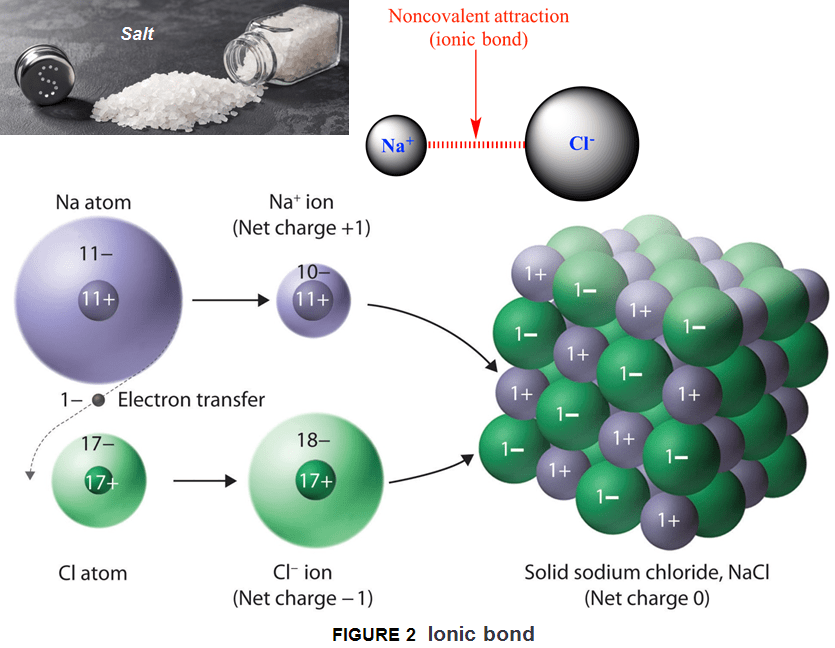
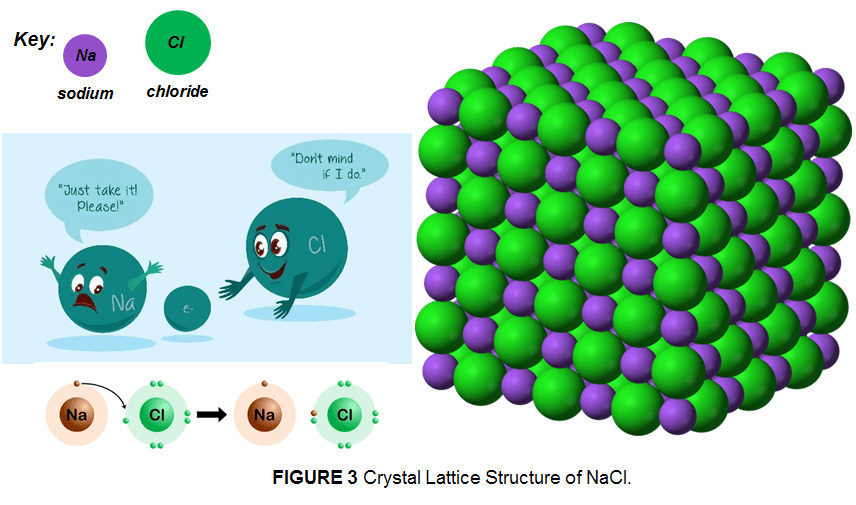
Ionic solids generally occur as crystalline solids having a 3d-arrangement of particles in a crystal lattice. As the anions line up, the cations accommodate in a way that they surround the anions. This arrangement helps to maximize the electrostatic attraction between the ions. With other words the ions bind with each other in a way that the overall compound is neutral (no negative or positive charge). Here, the number of cations surrounding an anion and vice versa can differ from one solid to another depending on the charge of involved cation and anion.
The easiest way to understand this is by looking at the crystal lattice structure of sodium chloride (NaCl-table salt). When we analyze the structure of sodium chloride (NaCl) we know that sodium ion (Na) has a charge of +1 so we called it the cation, whereas chlorine ion (Cl) has a charge of -1 and is called the anion. The metal atom transfers (donates) its electrons (becoming a cation) to the non-metal atom (which becomes a anion). So, in the crystal lattice structure of NaCl, the cation surrounds the anion on all sides. After that comes the next “layer” (or cube to be specific) of anions, then cations, and so on to build a crystal.
However, there are a couple of exemptions to this rule. In K (potassium), Rb (rubidium), Cs (cesium) oxides, the cation is the bigger one and as result the compound is a superoxide. Of course, this does not matter since then the anions will arrange to accommodate the cations anyway. The reason why these superoxides occur is because they have a relatively high number of electrons in their outermost shell, which makes them highly reactive. This makes it easy for them to gain an extra electron, creating a superoxide ion. Other elements may not form superoxide as easily because they have a lower number of electrons in their outermost shell, or because they are less reactive and therefore less likely to gain an extra electron. Additionally, some elements may not have the necessary chemical properties to form superoxide at all.
Besides, if the metal cation is not one metal atom but multiple ones ( such as Hg2+2-mercurous ion) joined together or have different atoms coordinated it will vary in size vastly. And just to complicate things, there are cations that are not metals, such as NH4+-ammonium ion.
Furthermore, the ionic solids containing hydrogen ions (H+) are acids, and those containing hydroxide ions (OH–) are bases. Ionic solids containing none of these ions are termed as salts. Salt compounds form from acid-base reactions. Besides, ionic solids may also form from evaporation (removing the solvent will crystallize the ions into a solid), precipitation, solid-state reactions, etc.
EXAMPLES OF IONIC SOLIDS.
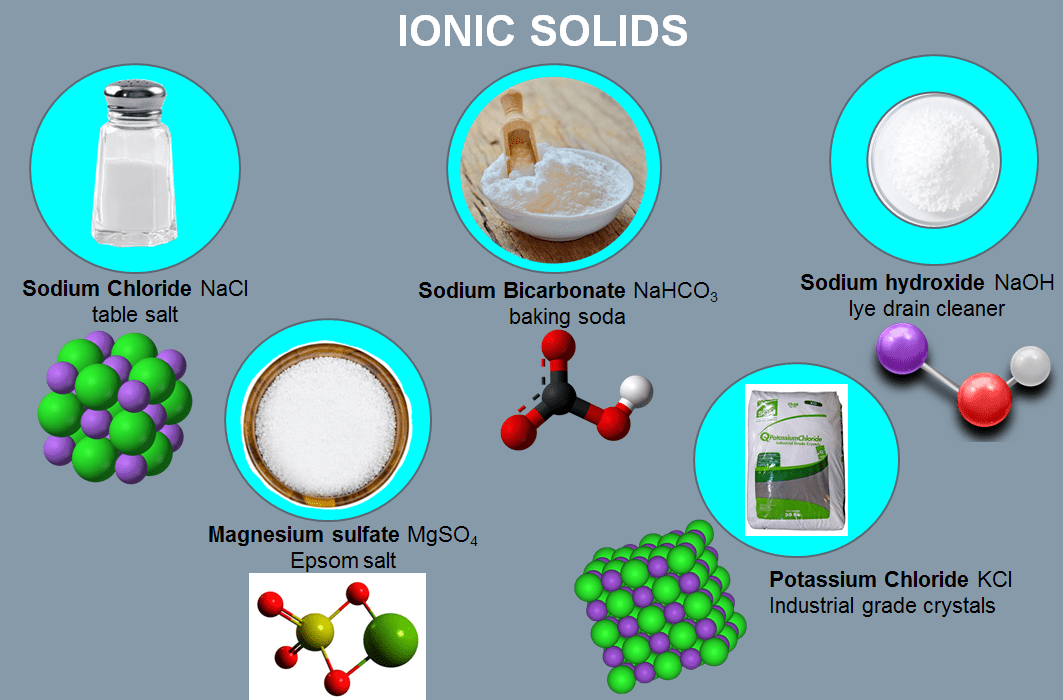
Table salt (sodium chloride, NaCl) is a common ionic solid, as is just about anything that is called a “salt”. Simple salts usually consist of one metal ion and one non-metal ion. Salts can also consist of more complex ions, such as ammonium sulfate, whose components ammonium (NH4+) and sulfate (SO42-), are individually held together by covalent bonds and attracted to each other through ionic bonds. They may also be composed of polyatomic ions such as NH4NO3 (ammonium nitrate) or AgClO3 (silver chlorate). Polyatomic ions are groups of atoms that share electrons (called covalent bonding) and function in a compound as if they constituted a single charged ion.

Many simple compounds formed by the reaction of a metallic element with a nonmetallic element are ionic.
PROPERTIES OF IONIC SOLIDS.
Ionic bonding is a strong electrostatic interaction and this is responsible for many of the properties of ionic solids. When describing the properties of ionic solids, we need to consider the following characteristics: the strength of electrostatic interaction, melting point, hardness, conductivity, solubility and lattice energy.
ELECTROSTATIC INTERACTIONS : As mentioned earlier, ionic solids are made up of ions held together by ionic bonds. These ionic bonds are considered a strong electrostatic interaction, and it affects the melting point and the other properties of ionic solids. The charges of ions also determine the strength of the attractive forces as stated by Columbus Law which says that: “the strength of the ionic bond is directly proportional to the charges on the ions”. Basically, the higher the charge of ions, the stronger the electrostatic attraction forces between them and the larger the coulombic forces.
MELTING POINT: Having strong ionic bonds means that a lot of kinetic energy is needed to break the bond between ions. The stronger the attractive forces between ions, the higher the melting point of ionic solids. Therefore most ionic solids have high melting points. (often between 300 and 1,000°C or even more).
For instance, let’s consider the charges of MgO and NaCl. Mg has a +2 charge, and O has a -2 charge, whereas Na has a charge of +1 and Cl has a charge of -1. So, based on the charge, we can say that MgO will have stronger attractive forces, and therefore a higher melting point. This is actually true, as the melting point of magnesium oxide (MgO) is 2852 °C and of sodium chloride (NaCl) is 801°C. However generally compared with covalent network and metallic bonds, the ionic bond is weaker and as result the ionic solid tend to have lower melting points than CN Solids or Metals.
VAPOUR PRESSURE: The ionic solids also have low vapour pressure which is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with temperature.
Vapor pressure is the equilibrium pressure of a vapor above its liquid or solid state in a closed container.
In this type of closed system, some molecules of a liquid or solid have enough kinetic energy to escape at the surface and enter the vapor (gas) phase. Meanwhile, some vapor molecules collide with the liquid or solid surface and change their phase. The kinetic energy of vapor molecules causes them to hit the walls and lid of a container, producing vapor pressure.A substance with a high vapor pressure is said to be volatile. Having low vapor pressure means that the ionic solid are not volatile, they don’t evaporate easily.
HARDNESS and BRITTLENESS: For the same reason, namely a fixed 3D directional structure, ionic solids are also typically of hard texture. Although they are hard, they also tend to be brittle, and when subjected to tensile stresses they shatter into pieces rather than bend.
ELECTRICAL CONDUCTIVITY : Ionic solids in their regular state as dry crystals are poor conductors, therefore can be good electrical insulators. However when ionic solids are heated until in their molten (liquid) state, or if they are dissolved in water (becoming a solution), they become good conductors of electricity. That’s because, in this state, the ions are released from the ionic bond, allowing electricity to pass through! The compounds whose aqueous solution or molten stale is able to conduct electricity are known as Electrolytes. Therefore ionic solids are likewise considered strong electrolytes.
THERMAL CONDUCTIVITY: When it comes to heat conduction, ionic solids are not the best solids for the job. For example ionic solids like sodium chloride (table salt) NaCl have strong electrostatic forced holding their molecules together and as result they are poor conductors of heat.
SOLUBILITY: by definition solubility is the ability of a solute to dissolve in a solvent in order to form a solution.
In case of Ionic solids we have a variety of cases. The temperature and strength of the ionic bonds are directly proportional to the solubility of ionic solids. To dissolve ionic solids, the ionic bonds between the atoms must be broken.
Ionic solids can easily dissolve in polar solvents such as water. Each atom or molecule within an ionic solid carries a charge and water molecule also carry a charge due to its polarity. As result the negative charges within water are attracted to the positive charged ions within the ionic solids and the positive charges within water are attracted to the negative ions in the ionic solid.This allows the water molecules to dissolve ionic solids by separating the parts, essentially trading the favorable ionic interaction in the solid crystal with favorable ionic interactions between the individual ions and the water molecules.Therefore, most salts are relatively water-soluble.
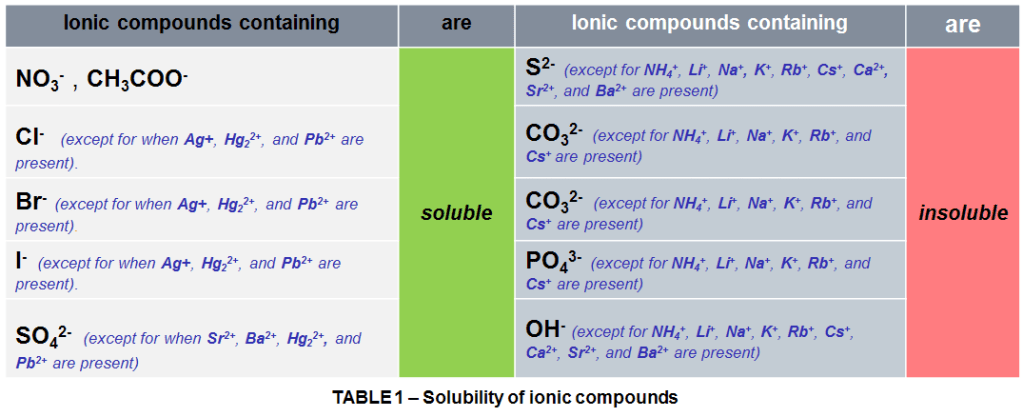
However, keep in mind that not all ionic solids are water-soluble (or other polar solvent). To know whether a compound will be soluble or insoluble, it’s helpful to take a look at which compounds are involved, by following the solubility rules as shown in Table 1. The temperature and strength of the ionic bonds also affect the solubility of solids being direct proportional.
LATTICE ENERGY: The lattice structure of ionic solids consists of a well-arranged pattern, with cations surrounding the non-metal anion. The stability of the crystal lattice depends on the strength of the attractive forces between the oppositely charged ions. The stronger the attraction, the higher the stability of the crystal lattice and the greater the lattice energy.
Lattice energy is defined as the energy released when an ionic solid is formed from its ions.
This energy can be used to estimate the strength of ionic bonds. The greater the lattice energy, the stronger the ionic bond and of course it depends on the charge of the ions and on the size of the ions, so we’ll have the following 2 situations:
- The greater the charge of the ions, the greater the lattice energy (⇧ charge of the ions = ⇧ lattice energy)
- The smaller the size of the ions, the greater the lattice energy ( ⇩ size of the ions = ⇧ lattice energy)
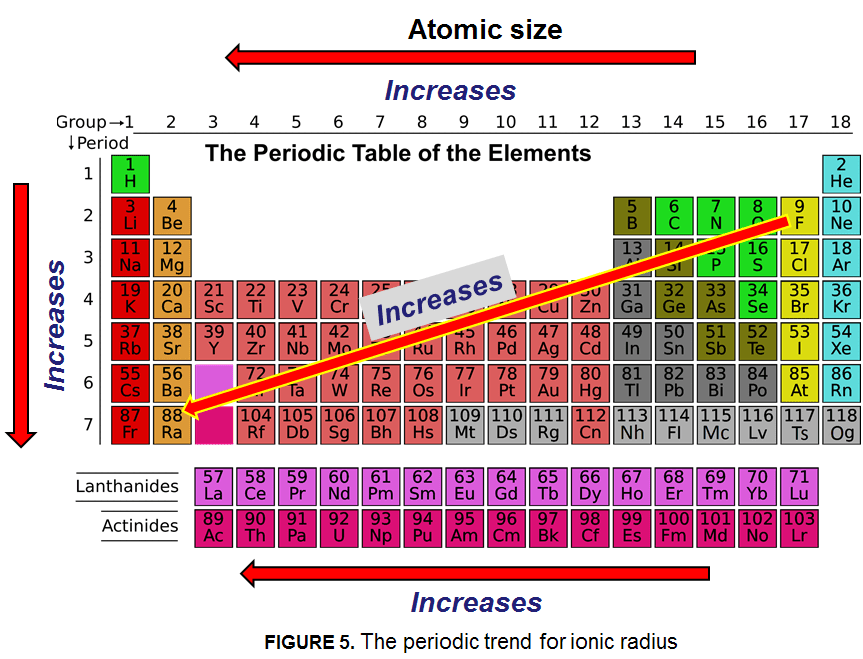
The trend for ionic radius is that ionic radius increases as the number of electrons increases. Francium (Fr) has the largest atomic size on the periodic table, and helium (He) has the smallest atomic size. The atomic radius is measured as half the distance between two nuclei of the same atoms that are bonded together. The periodic trend for ionic radius is shown below:
Leave a comment