From all the metals humans ever used non of them created a revolution in technology at the scale Iron did. This metal became the reference element in metallurgical studies for other metals as well. If you understand Iron you actually understand not only metals but you understand the entire material science engineering. In this post I want to talk about it, for everyone who intend to start in materials science this is the 1st metal you will need to study and understand. It is exactly how I also started to study materials. Iron was the first one for me too. Let’s see what’s its story.
The global economy has grown by leaps and bounds in the last couple of centuries and steel has had an indispensable role in this development. But what do we know of its origins?
To know steel, we must first understand iron (Fe)(from latin = ferrum), for these two metals are nearly one and the same. Steel contains 98-99% of iron concentration or more. The small remainder is carbon (C), which makes a major difference in the metal’s properties. Now let’s go back a little in time to know how it all started.
It is an odd fact that steel was not understood by science until the 20th century. Before that, for thousands of years, the making of steel was handed down through the generations as a craft. Even in the 19th century, when we had an impressive theoretical understanding of astronomy, physics and chemistry, the making of iron and steel on which our Industrial Revolution was based was achieved empirically – through intuitive guesswork, careful observation and a huge slice of luck. During the Stone Age, metal was extremely rare and highly prized, since the only sources of it on the planet were copper (Cu) and gold (Au), which occur naturally, if infrequently, in the Earth’s crust (unlike most metals, which have to be extracted from ores). Some iron (Fe) existed too, most of it having fallen from the sky in the form of meteorites.
In the absence of copper, gold and meteoric iron, our ancestors’ tools during the Stone Age were made of flint, wood and bone. Anyone who has ever tried to make anything with these kinds of tools knows how limiting they are: if you hit a piece of wood it either splinters, cracks or snaps. The same is true of rock or bone. Metals are fundamentally different from these other materials because they can be hammered into shape: they flow, they are malleable. Not only this, they get stronger when you hit them; you can harden a blade just by hammering it. And you can reverse the process simply by putting metal in a fire and heating it up, which will cause it to get softer. The first people to discover these properties 10,000 years ago had found a material that was almost as hard as a rock but behaved like a plastic and was almost infinitely reusable. In other words they had discovered the perfect material for tools, and in particular cutting tools like axes, chisels and razors. This ability of metals to transform from a soft to a hard material must have seemed like magic to our ancient ancestors.
In the late 18th century, during the Industrial Revolution in England, the invention of the steam engine by James Watt enabled the blasting of air into the blast furnace with a machine. This made the mass production of iron possible. Although iron was the main driver of this revolution, it was by no means a new material. It had been around for nearly 3000 years since the Iron Age. For many centuries, the British had converted their iron ores to iron & steel by heating the raw material with charcoal obtained from trees.
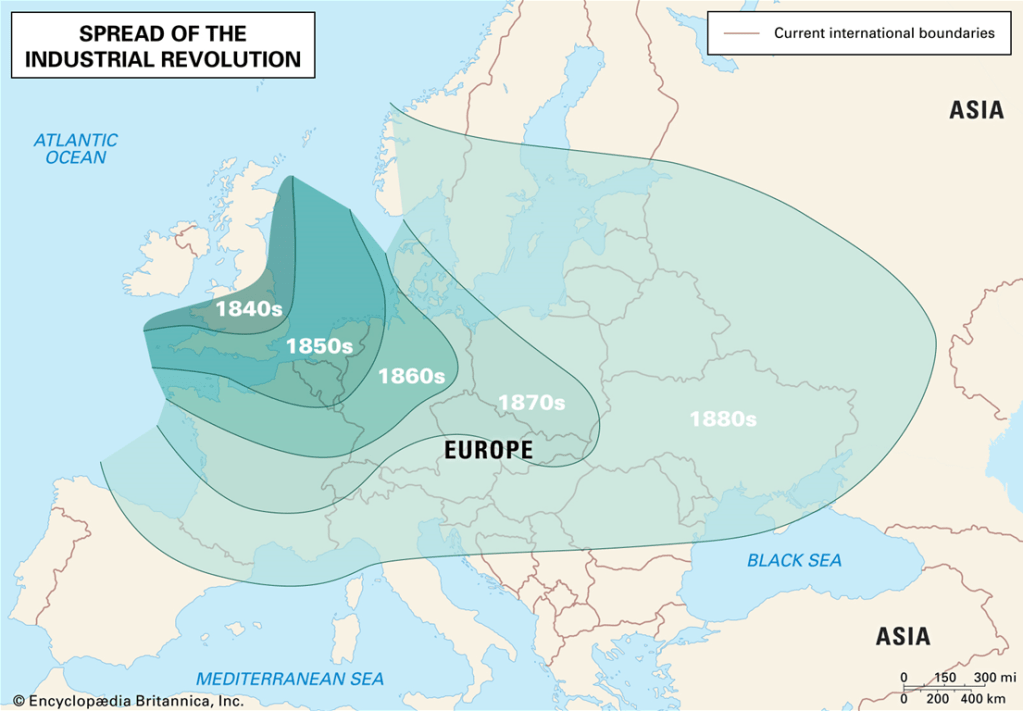
By the mid-18th century, however, the iron industry was in a downward trajectory. Iron workers required charcoal to smelt the iron ore, but charcoal was in short supply as the wood required to make charcoal was expensive. This posed an industrial problem. What was now required was a method by which iron could be smelted in high tonnage quantities. It needed a better heat source than charcoal, possibly a new fuel for treating iron ores.
The fuel they found was coal. In the course of a century, substantial change occurred as coal replaced charcoal as the fuel for smelting process. Coke proved to be a far superior material for converting iron ore to iron and then steel. It was obtained by heating coal in the absence of air. The materials were now cost efficient and sufficient in supply. The result of this change revolutionized the industry and the use of iron and steel.
The conversion of the iron and steel from charcoal to coke was accompanied, however, by several new technical problems, like the high concentration of sulfur (S) in coal, which along with other impurities makes iron brittle. Steel was a stronger and less brittle than iron, but it was more difficult to make. This encouraged the development of even more new inventions. Along the way, a key discovery was that the amount of carbon present in the iron controlled not only its melting point but also its properties. By controlling the additions of carbon (C) through the use of coke, a form of iron was made called steel which could be cast on an industrial scale.
At the beginning of 20th century steel was already notorious for the ability to keep its shape so it was started to be used for razor blades which were first introduced by the American inventor King Camp Gillette in 1901. In fact daily shaving was not a widespread practice in the 19th century so some people never shaved. The custom of shaving every day among American men is a 20th-century innovation which was started after World War I. During this time these razor blades indeed proved that they are very useful and the next idea that came up was to build a kind of machine which could be used to sharpen these blades easier. But here things are not that simple as they seem. To build such a machine and to effectively measure the resulted sharpness we must have a real appreciation about the physics and chemistry taking place during the sharpening process.
The reason is that metals are made from crystals, the average razor blade contains billions of them, and in each of these crystals the atoms are arranged in a very particular way, a near-perfect three-dimensional pattern.

The bonds between the atoms hold them in place and also give the crystals their strength. A razor gets blunt because the many collisions with hair that it encounters force bits of these crystals to rearrange themselves into a different shape, making and breaking bonds and creating tiny dents in the smooth razor edge. Resharpening a razor through some electronic mechanism, would have to reverse this process. In other words, it would have to move atoms around to rebuild the structure that had been destroyed.
To sharpen a piece of something that means you must remove a layer of material form it and this action generates heat. This heat, whether electrically produced or not, usually has a different effect on the material, it can change its atomic structure and it always softens metal crystals, therefore the sharpening process must avoid excessive heating.
The ages of civilization, from the Copper Age to the Bronze Age to the Iron Age, represent a succession of stronger and stronger alloys. Copper is a weak metal, but naturally occurring and easy to smelt. Bronze is an alloy of copper, containing small amounts of tin (Sn) or sometimes arsenic (As), and is much stronger than copper. So, if you had copper and you knew what you were doing, for very little extra effort you could create weapons and razors 10 times stronger and harder than copper. The only problem is that tin and arsenic are extremely rare. Elaborate trade routes evolved in the Bronze Age to bring tin from places such as Cornwall and Afghanistan to the centres of civilization in the Middle East for precisely this reason.
Modern razors too are made from an alloy but, it is a very special sort of alloy, the existence of which puzzled our ancestors for thousands of years. Steel, the alloy of iron (Fe) and carbon (C), is even stronger than bronze, with ingredients that are much more plentiful: pretty much every bit of rock has some iron in it, and carbon is present in the fuel of any fire. Our ancestors didn’t realize that steel was an alloy – that carbon, in the form of charcoal, was not just a fuel to be used for heating and reshaping iron but could also get inside the iron crystals in the process. Carbon doesn’t do this to copper during smelting, nor to tin or bronze, but it does to iron. It must have been incredibly mysterious – and only now with a knowledge of quantum mechanics can we truly explain why it happens (the carbon in steel doesn’t take the place of an iron atom in the crystal, but is able to squeeze in between the iron atoms, creating a stretched crystal).
There is another problem, too. If iron becomes alloyed with too much carbon – if, for instance, it contains 4% carbon instead of 1% carbon – then it becomes extremely brittle and essentially useless for tools and weapons. This is a major obstacle because inside a fire there is rather a lot of carbon around. Leave the iron in too long, or allow it to become liquid in the fire, and a huge amount of carbon enters the metal crystals, making the alloy very brittle. Swords made from this high-carbon steel snap in battle.
Until the 20th century, when the alloying process was 1st fully explained, no one understood why some steel-making processes worked and others didn’t. They were established by trial and error, and those that were successful were handed down to the next generation and were often trade secrets. But even if they were stolen, they were so complicated that the chances of successfully reproducing someone else’s steel-making process were very low. Certain metallurgical traditions in certain cultures became known for making extremely high-quality steel-and such civilizations thrived.
