The most representative group of elements that makes Nanotechnology available is the Group 14 (or IV A) in the Periodic Table. This group is known as The Carbon Group and it includes 6 elements in the following order from top to bottom as follows:
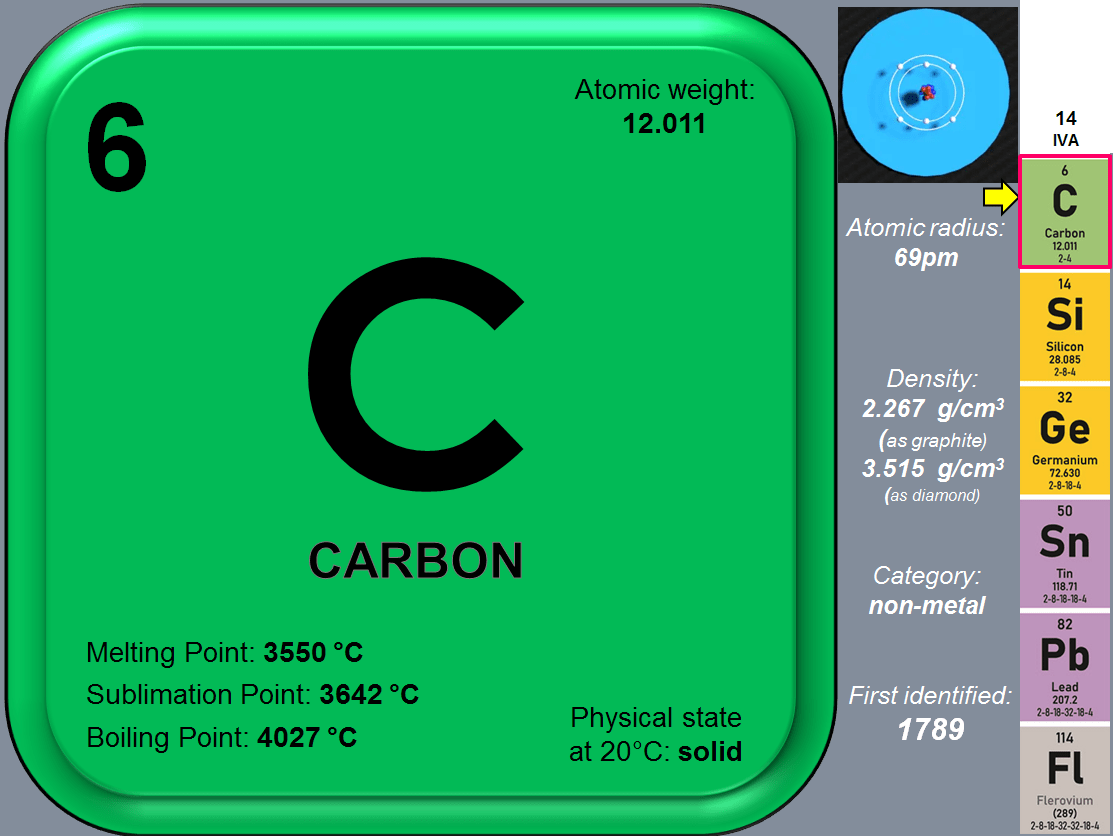
- Carbon (C) – the element 6
- Silicon (Si) – the element 14
- Germanium (Ge) – the element 32
- Tin (Sn) – the element 50
- Lead (Pb) – the element 82
- Flerovium (Fl) – the element 114
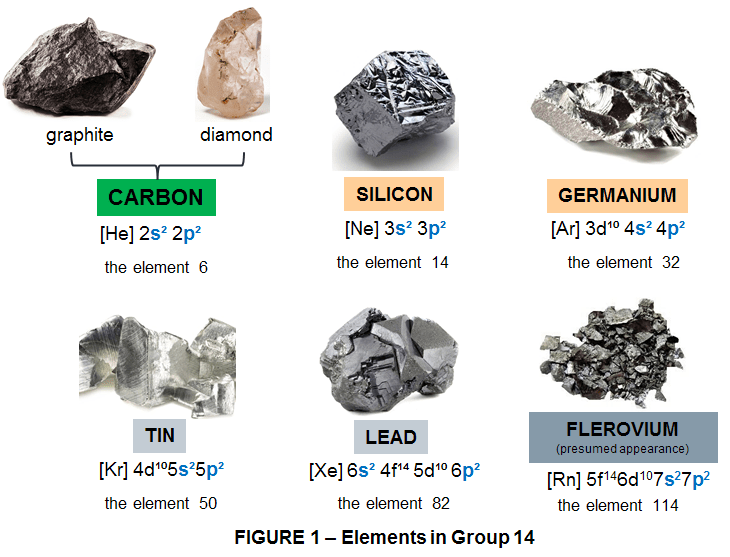
In their elemental state, these 6 elements vary considerably: Carbon is a nonmetal, black (in its graphite form) or transparent (in its diamond form). Silicon and Germanium are semiconducting metalloids with silvery-blue or dark gray color and a metallic luster; Tin and Lead are shiny gray metals, and Flerovium appearance is currently unknown because only a few atoms have ever been created, but it is presumed to be a metallic, silvery-white, or gray solid. They all form a wide variety of compounds and have numerous applications (highly representative for nanotechnology being C, Si, Ge and Sn). Except the element 114 (Fl) all the other 5 in this group, occur naturally in Earth’s crust.
What makes these elements special is their electronic configuration at atomic level. All these 6 elements have 4 electrons in their outer shell, with a configuration ending in s2p2 . That means the external layer of electrons is half-filled and this is the origin of very interesting chemical features, especially in the case of Carbon. All elements in group 14 have conduction bands, which are energy levels in which electrons can move freely between atoms, in a so called “electronic soup”. This property is characteristic of metals. The further you go down the group, the more easily electrons move through the conduction band. And this is very interesting because as I mentioned earlier not all 6 elements in this group are metals.
Carbon for example, in general, is not a good conductor of electricity, although in the form of graphite it can do so quite well. Silicon and Germanium are classic semiconductors: they can be made to conduct electricity if their electrons are given an extra dose of energy, either in the form of light or heat. Tin and Lead are true metals: and some of their electrons always remain in the conduction band, making both Sn and Pb good thermal and electrical conductors. Regarding Flerovium while its conductivity is not yet confirmed, due to its atomic instability, its classification as a metal suggests it is a good conductor, similar to other metals in its group. Let’s now focus on the first element in this group: CARBON. Its chemical symbol is noted as: C. Called in different languages its name is:
Carbo (in latin); Carbon (in english); Carbon (in romanian) ; Carbón (in spanish); Carbone (in french); Kohlenstoff (in german), Koolstof (in dutch).
WHAT IS CARBON?
The element Carbon is very common in the universe coming in as the 4th most abundant element by mass (after H, He and O). About 0.5% of the universe is carbon. That’s 1 atom out of every 200. It’s also the 4th most abundant element in the Sun, making up 0,3% or about one in 300 atoms. Likewise, carbon is the 7th most abundant element in meteorites (after O,Fe,Si,Mg,Sn and H). Carbon is 1.5% of their makeup. There’s a whole class of meteorites called carbonaceous chondrites that can contain up to 3% carbon. In Earth’s crust, carbon makes 0,18%, that’s 1 in 550 atoms, which make is the 10th most abundant element. Also Carbon is the 9th most abundant element in the oceans at about 1 out of every 36000 atoms which is only 0.002%, but that’s still a lot compared to other elements. Actually the oceans hold about 40,000 billion tonnes of carbon, primarily as dissolved inorganic carbon like bicarbonate ions. This represents a carbon reservoir 50 times larger than the atmosphere and is constantly exchanging with it. The majority of carbon in the ocean is in inorganic forms, with bicarbonate (HCO3−) being the most abundant species, followed by carbonate (CO32−) and dissolved carbon dioxide (CO2). Likewise the ocean absorbs about 30% of the carbon dioxide that is released in the atmosphere.
And lastly and maybe not surprisingly being a carbon-based life form Carbon is the 2nd (after Oxygen) most abundant element in us making up 23% of our body’s mass.
11 elements were discovered prehistorically; certainly carbon tops this list with its appearance every time something is burned; However that doesn’t mean it was recognized as an element prehistorically; is one of those elements discovered prehistorically because it sits in its native form as coal and graphite in many places on Earth. Of course it also occurs naturally as Diamond but perhaps not as easily found.
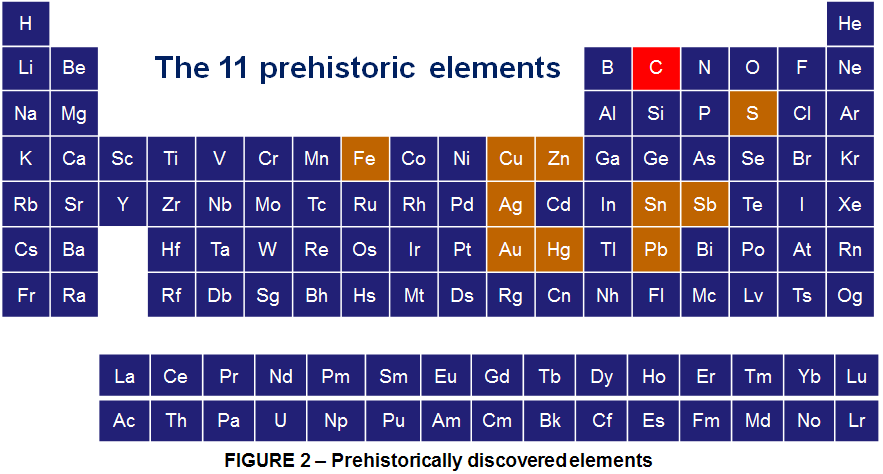
Carbon was discovered in prehistory and was known in the forms of soot and charcoal to the earliest human civilizations. Diamonds were known probably as early as 2500 BCE in China, while carbon in the form of charcoal was made by the same chemistry as it is today, by heating wood in a pyramid covered with clay to exclude air. However the Carbon was first recognized as an element by French chemist Antoine Lavoisier in 1789.
TYPES OF CARBON
Carbon is the 6th element in the Periodic Table because its atomic number is 6, because that’s how many protons and neutrons are in its nucleus. And that is what distinguishes it as a unique element; The number of protons is always 6 yet it may happen that sometimes there can be different numbers than 6 of neutrons in the nucleus. All these different atomic forms are called Isotopes. They’re chemically identical to each other, but with slightly different weights and physical properties.
As of today there are 15 known isotopes of Carbon. Of these 15, there are only 2 stable non-radioactive isotopes: Carbon-12 (12C) and Carbon 13 (13C).
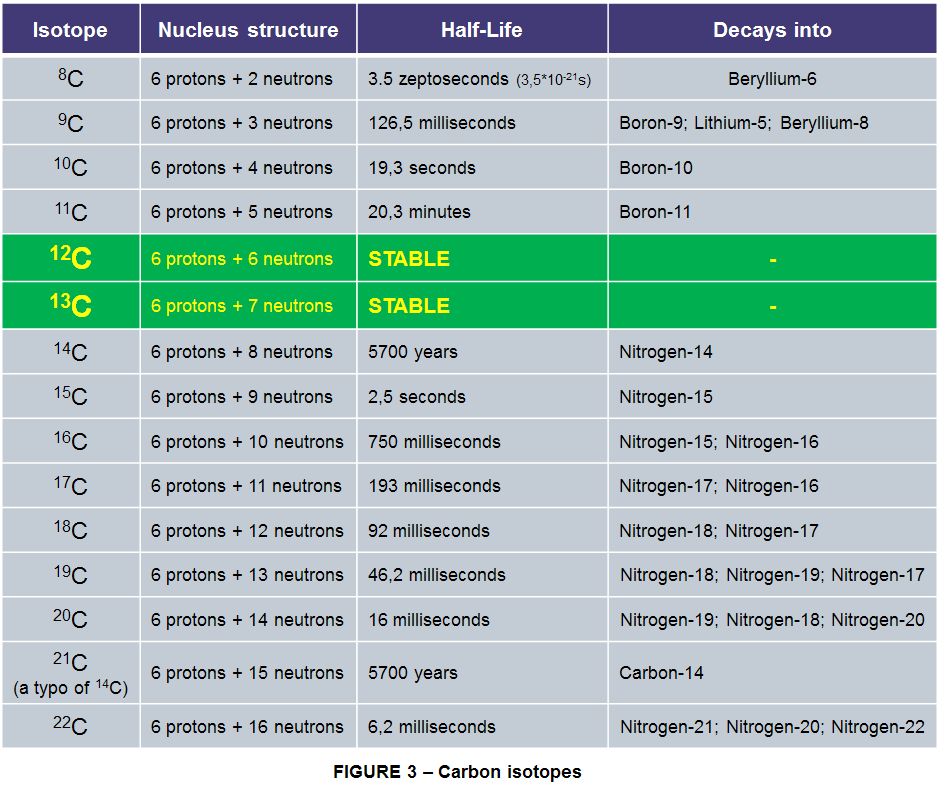
12C makes up the vast majority of carbon in the universe 98.93% of it. The remaining 1.07% is the slightly heavier 13C.
There is also the unstable Carbon-14 (14C) which is the longest-lived radioactive isotope with a half-life of 5700 years. The most stable artificially created is the Carbon-11 (11C) isotope with a half-life of around 20 minutes and the rest of other 11 carbon isotopes are short lived under 20 seconds, most having a half-life of even less than 200 milliseconds.
WHERE DOES CARBON COMES FROM?
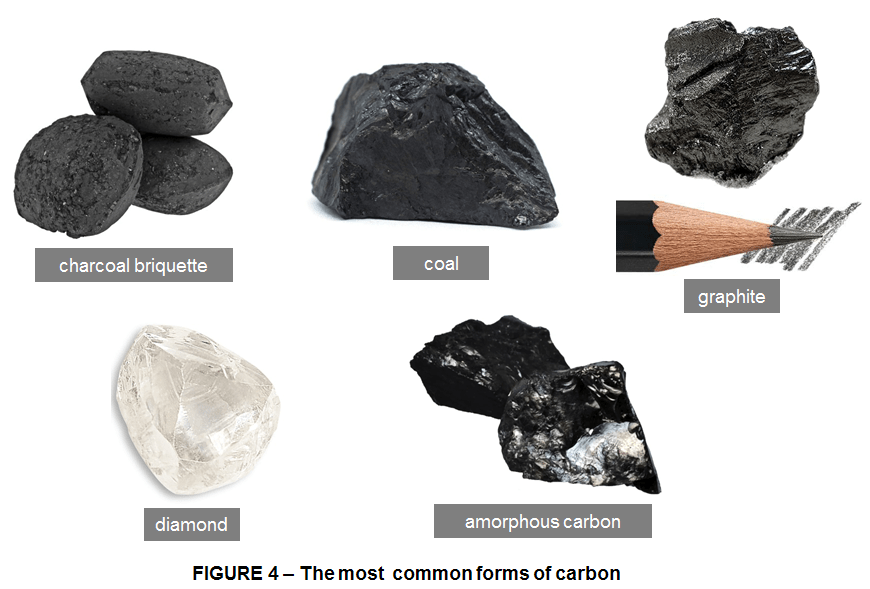
As the 10th most abundant element Earth’s crust, Carbon appears mainly in the form of carbonate minerals, such as limestone (CaCO3), and fossil fuels, such as coal. Carbon’s ability to form hybrid orbitals makes it a very versatile element; there are more carbon compounds than all other elements combined can create. Combined with other elements or in its pure form Carbon is wonderfully diverse. Pure carbon takes on several forms or allotropes. The main allotropes of carbon are diamond, graphite, graphene, amorphous carbon, and a series of substances called fullerenes.
Therefore since carbon is such a common element it can be easily found in many different forms. It can be in the form of a charcoal briquette that you might use in your barbecue or carbon in the form of coal, or you might have it as graphite in your pencil. Carbon it can be found even in a crystalline form such as diamond.
Life as we know it depends on carbon chemistry, and all living things discovered so far are carbon-based life forms. Because carbon compounds are essential to all living organisms, carbon chemistry is also known as organic chemistry. However, organic compounds also form the basis of the petrochemical industry, which includes plastics, dyes, adhesives, and synthetic solvents.
Carbon is present in all existing systems on planet Earth: in the biosphere (all living beings), in the atmosphere, in the lithosphere (Earth’s crust) and in the hydrosphere (rivers, lakes and oceans). The successive and constant exchange of carbon between these systems is called the carbon cycle. The main component of this cycle involves the absorption of carbon dioxide from the atmosphere during photosynthesis and its subsequent release back into the atmosphere through respiration. Carbon dioxide is also released when living things burn. When an organism dies, it normally decomposes; during this process, its carbon content is released into the atmosphere as carbon dioxide or methane. In some circumstances, organisms do not decompose. The world’s tropical forests store more than 200 billion tons of carbon, which trees and other plants have extracted from atmospheric carbon dioxide (CO2) through photosynthesis.
This is a topic that is close to my heart… Many thanks! Where are your contact details though?
LikeLike
I am glad to hear. 🙂 For my contact details just take a look on my website, in the top menu, there is a tab with my contact details 😉
LikeLike