The far right column in The Periodic Table is the group 18th of elements also known as the group of noble gases. It includes 7 elements in the following order from top to bottom as follows:
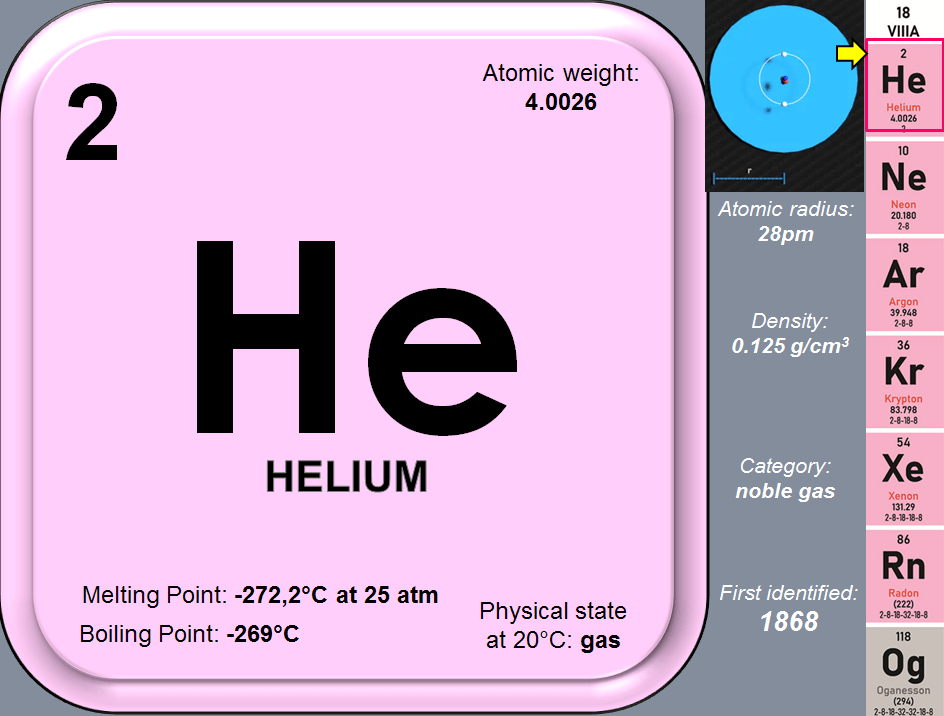
- Helium (He)-element 2
- Neon (Ne)-element 10
- Argon (Ar)-element 18
- Krypton (Kr)-element 36
- Xenon (Xe)-element 54
- Radon (Ra)-element 86
- Oganesson (Og)-element 118
All these are inert gases, (non-reactive), which means that naturally they don’t bond with other elements in order to form compounds. They can be somehow forced to be reactive and form compounds, but only through special processes although this is quite difficult. Except the element 118 (Og) all the other 6 in this group, occur naturally and they were all 1st discovered and isolated in the last decade of 19th century by the Scottish chemist William Ramsay.
Inert means also that they are mostly non-toxic, however the last 2 in the group are. For instance Radon (Ra) as the heaviest natural noble gas, is highly radioactive and Oganesson (Og) like all artificially created elements is radioactive by default. By far the most representative element among noble gases is: HELIUM. Its chemical symbol is noted as: He. Called in different languages its name is:
Helium (in latin); Helium (in english); Heliu (in romanian) ; Helio (in spanish); Hélium (in french); Helium (in german), Helium (in dutch).
WHAT IS HELIUM?
Nearly everything in the universe that isn’t Hydrogen, is HELIUM – all the other elements make up only about 2% of the mass of the universe, in spite of being heavier than the 2 lightest, simplest elements (H and He). Helium does make up about 24% of the mass of the sun; in the extreme temperatures of our star, hydrogen nuclei undergo a process of fusion and helium is formed. Being the 2nd most lightest element after Hydrogen, a helium atom is made of 2 protons (and 2 neutrons) in its core (nucleus) and 2 electrons orbiting the core.

Noble gases are inert because they all have complete electron shells; this is also the reason why they appear on the far right of the periodic table. The atoms of these elements are so inert that they do not even bond with themselves. Unlike the group 17th (the halogens), which exist as diatomic molecules such as F2yCl2, the elements in group 18th are all mono-atomic. They all have very low boiling points, remaining in gaseous state until they are cooled to very low temperatures. The boiling point is known to be related to the atomic mass, therefore it increases as you go down the table. Hence, Helium has the lowest boiling point among all the elements at -269°C, while that of radon is -61°C. The atoms of the group 18 elements are spherical, a trait that only applies to atoms that have full or half-filled orbitals.
Helium was one of two elements that formed in large quantities during the first minutes of the Universe (the other element was hydrogen). What actually originated in our early solar system were helium nuclei, not helium atoms. According to many astronomical studies it is now largely accepted that there were no atoms of any kind until about 300,000 years after the Big Bang, a period known as “the epoch of recombination” when the temperature of the Universe fell enough for electrons to combine with nuclei. The rest of the elements appeared much later, in stars and in gigantic supernova explosions.
Given that large quantities of helium were created just after the Big Bang and stars are constantly producing more, it is not surprising that this element is the second most abundant in the Universe (after hydrogen). Almost 1 in every 10 nuclei or atoms in the universe is helium.
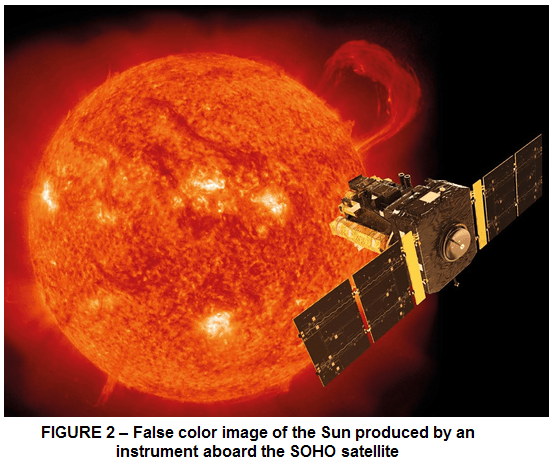
As shown in Fig. 2 through this false-image of Sun, created by an instrument aboard the Solar Heliospheric Observatory (SOHO) satellite, we can see that the instrument detects ultraviolet radiation emitted by helium ions in the sun’s chromosphere, a layer that is normally invisible due to the influence of the outer layer, the photosphere.
TYPES OF HELIUM
As of today there are 9 known isotopes of Helium. Yet only 2 of them are stable: Helium-3 (3He)(with a nucleus of 2 protons and 1 neutron) and Helium-4 (4He)(with a nucleus of 2 protons and 2 neutrons).
4He is an unusually stable nucleus because its nucleons are arranged into complete shells. 4He is therefore really the only version of the element we experience here on Earth, making up roughly 99,99986% of the planet’s entire helium supply. It occurs by alpha decay of heavier radioactive elements; Unfortunately this Earth abundant version of Helium isn’t the version that’s making headlines, instead the golden child is a separate isotope 3He. Equal mixtures of liquid 3He and 4He below 0.8 K will separate into two immiscible phases due to their dissimilarity (they follow different quantum statistics: 4He atoms are bosons while 3He atoms are fermions).
The other 7 are a subset of exotic light nuclei which have larger atomic masses than helium’s natural isotopes and are only used for scientific research. These are:
- Helium-2 (2He) with half-life = < 10-9 seconds; -nucleus of 2 protons; 0 neutrons
- Helium-5 (5He) with half-life = 6.02(22)×10−22 s; -nucleus of 2 protons; 3 neutrons
- Helium-6 (6He) with half-life = 806.92(24) ms;-nucleus of 2 protons; 4 neutrons
- Helium-7 (7He) with half-life = 2.51(7)×10−21 s; -nucleus of 2 protons; 5 neutrons
- Helium-8 (8He) with half-life = 119.5(1.5) ms;-nucleus of 2 protons; 6 neutrons
- Helium-9 (9He) with half-life = 2.5(2.3)×10−21 s;-nucleus of 2 protons; 7 neutrons
- Helium-10 (10He) with half-life = 2.60(40)×10−22 s;-nucleus of 2 protons; 8 neutrons
Although all the 7 exotic helium isotopes decay with a half-life of less than one second, researchers have eagerly created exotic light isotopes through particle accelerator collisions to create unusual atomic nuclei for elements such as helium (He), lithium (Li), and nitrogen (N). The bizarre nuclear structures of such isotopes may offer insight into the isolated properties of neutrons. The helium nuclei formed in the early days of the Universe included the two stable isotopes of helium: 3He and 4He. Both isotopes continue to constantly form within each active star, as a result of nuclear reactions in which hydrogen nuclei fuse. These fusion reactions are the main source of energy in stars, which results in photons which in turn generate light, and obviously that’s exactly why we can see the stars.
WHERE DOES HELIUM COMES FROM?
There is plenty of Helium in the Universe. The glowing nebula (cloud of gas and dust) as shown in Fig.3 is the Crescent Nebula. It is so vast that our entire Solar System would fit inside 7 times over. The nebula‘s light comes from a super-heated star at its centre known as WR 136, this star is 15 times heavier than out Sun and 250.000 times bigger. It‘s immense power comes from its fuel = Helium.

Helium makes the Star WR 136 hot and bright. This star once burned using hydrogen like our Sun. Hydrogen atoms smashed together in the stars‘s core until they became helium atoms releasing energy in the process. However the star ran out of hydrogen about 200.000 years ago. It began smashing together helium atoms instead and ballooned into a gigantic red star, sending out a cloud of gas that spread around it. The star is producing a wind of electrified gases that hurtles out at 1700 km every second. This wind continues to crash into the gas cloud making it grow into the nebula we see. Eventually WR 136 will run out of helium and its other fuels and explode into an enormous fireball called supernova. However, Helium is unusual in that its isotopic abundance varies greatly depending on its origin. In the universe as a whole, 4He nuclei outnumber 3He nuclei by a ratio of 10,000 to 1.
On Earth, the helium in the air is concentrated in the outermost layers of the atmosphere. In general, approximately one in every 200.000 gas particles that make up the atmosphere is a helium atom, and from the 2 stable Helium isotopes in the Earth’s atmosphere, there is one 3He atom for every million 4He atoms. This primordial variation dates back all the way to our planet’s infancy forming in the mantle between earth’s core and crust. These days however this particular He isotope (3He ) is extremely hard to come by on the planet. 3He comes mostly from solar winds, but because the Earth is protected by our atmosphere and out magnetosphere these solar winds don’t really trickle down to us. One scientific report shows that only roughly 0,01 metric tons of 3He exist on Earth, and it comprises only 0,001% of the USA Helium reserves which I didn’t even know we had a helium reserve.
There is only a trace amount of 3He on Earth, primarily present since the formation of the Earth, although some falls to Earth trapped in cosmic dust. Trace amounts are also produced by the beta decay of tritium.In stars, however, 3He is more abundant, a product of nuclear fusion. Extraplanetary material, such as lunar and asteroid regolith, have trace amounts of 3He from being bombarded by solar winds. The different formation processes of the two stable isotopes of helium produce the differing isotope abundances. These differing isotope abundances can be used to investigate the origin of rocks and the composition of the Earth’s mantle.
As a result of its scarcity in the Earth’s atmosphere, helium is not extracted from the air, but from the bowels of the earth. It comes mostly as 4He and is a byproduct of the natural gas industry: the gas mixture extracted from wells typically contains a certain percentage of helium. This underground helium is formed by the degradation of radioactive elements in Earth’s crust. Several thousand tons are produced this way each year. Some are trapped in impermeable rock formations, the same traps where natural gas accumulates.
As one of the noble gases, helium is the least reactive known element, and unlike hydrogen, it isn’t extensively captured in compounds. However, just like hydrogen, helium in its pure form is lighter than air and prone to escaping the Earth’s atmosphere. Unlike nitrogen (N), which makes up 78% of the atmosphere, most helium atoms escaped into space early in Earth’s history. The less massive the particles of a gas are, the greater their average speed; In general individual light helium (He) atoms move much faster than for example the diatomic nitrogen (N2) molecules, which are much heavier. Those that did not come out initially, over time they managed to reach a sufficiently high speed, as result of collisions with other atoms and molecules.
In contrast to Earth, the giant gas planet Jupiter has maintained the majority of this element; Its atmosphere contains 24% helium. This is because the planet is colder and has a much greater gravitational pull. Within starts larger than planet Jupiter, helium nuclei fuse to make heavier nuclei. For example, each nucleus of the most common isotope of oxygen, Oxygen-16 (8p, 8n), is composed of 4 fused 4He nuclei. Some large nuclei are unstable, and one of the ways they gain stability is by shrinking, releasing a group composed of two protons and two neutrons, known as an alpha particle. These alpha-particles that emerge from such a process are in fact fully ionized 4He nuclei. So whenever radioactive substances undergo alpha decay, they create 4He nuclei. If an alpha particle gains 2 electrons it becomes a neutral helium atom.
Leave a comment