Usually, matter is viewed as existing in one of the 4 main state forms — solid, liquid, gas, or plasma—depending on the conditions under which it is studied.
In the solid state, substances are very orderly, with their particles (molecules, atoms, or ions) arranged in highly organized ways. Therefore in a solid, the atoms or molecules are tightly bonded together into a rigid shape. Particles in solids can vibrate about fixed positions, but they cannot move. So solids feel hard and keep their shape.
In the liquid state, the matter is less orderly and less tightly bonded than in solids. However, particles in liquids still possess some organization. Atoms or molecules in liquids have only weak bonds between them, so the particles can move around, they are able to rotate as well as vibrate. This means liquids are free to flow, but still tight enough packing of the particles prevents them from being compressed.
In the gas state the matter is even more disordered, and its disorganized constituent particles are widely separated from one another. In a gas, there are no bonds between the atoms or molecules, so they can freely spread out and fill their container. The particles are also far apart, so a gas can be compressed, although doing so increases the pressure.
In the plasma state, matter is similar to the gas state but is mainly defined as containing charged particles (as a cloud of free electrons and positively ionized atoms or molecules) that interact with one another and add up to no net electrical charge. This can be achieved by heating a gas to a very high temperature or by passing an electric current through it.
However, except these 4 most frequent states of matter of course there are many others. One of them of particular high interest is The Liquid Crystal state.
WHAT IS THE LIQUID CRYSTAL STATE?
The study of liquid crystals began in 1888 when the Austrian botanist named Friedrich Reinitzer observed that an organic material known as cholesteryl benzoate (often used in some hair-colors, make-ups and other cosmetics preparations) had 2 distinct melting points. In his experiments, Reinitzer increased the temperature of a solid sample from this material up to 145°C and watched the crystal change into a hazy liquid. As he increased the temperature further up to around 180°C, the material changed again into a clear, transparent liquid. When cooled, the liquid changed back to the liquid crystal state and then to a crystalline solid at appropriate temperatures. Reinitzer also noted that the chemical substance changed color from red to blue when heated and that the color change was reversed upon cooling. So, what exactly happened here?
A general explanation is this: The particles of many solids are arranged in very regular, repeating patterns. These solids are called crystalline solids and are said to exist as crystals. Usually, crystals are converted into liquids very smoothly by heating.In many organic chemicals, however, and in a few inorganic ones, the tendency toward an ordered structural arrangement of particles is so great that their crystalline form does not melt directly to a liquid form. Rather, it first passes through an intermediate form that is neither a liquid nor a crystal but possesses properties of both these state forms of matter, that means they may pour like liquids, but they can exhibit a semi-ordered state between that of a liquid disorder and crystal order, the substance in this case has a paracrystalline structure. When this happens, the substance is said to exist in the liquid crystal state. Therefore because of this early work, Reinitzer is often credited with discovering a new phase of matter – the liquid crystal phase.

The term “crystallinity” implies the presence of 3-dimensional order on the level of atomic dimensions. In polymers, the range of order may be as small as about 2 nm in one (or more) crystallographic direction(s) and is usually below 50 nm in at least one direction. Polymer crystals frequently do not display the perfection that is usual for low-molecular mass substances. Polymer crystals that can be manipulated individually are often called polymer single-crystals. To quantify just how much order is present in a material, an order parameter (S) is defined as follows:

S = (1/2) (3cos2 θ-1)
where theta (θ) is the angle between the director and the long axis of each molecule. The brackets denote an average over all of the molecules in the sample.Hence the term S describes the orientational order of liquid crystalline material, allowing for the individual orientational deviation of the molecules from the director, which represents the average over the collection. In an isotropic liquid, the average of the cosine terms is zero, and therefore the order parameter is equal to zero. For a perfect crystal, the order parameter evaluates to 1.
Typical values for the order parameter (S) of a liquid crystal range between 0.3 and 0.9, with the exact value a function of temperature, as a result of kinetic molecular motion.This is illustrated below for a nematic liquid crystal material. (Ordinary nematic liquid crystals are the least ordered type of liquid crystal, making them the most like a true liquid)
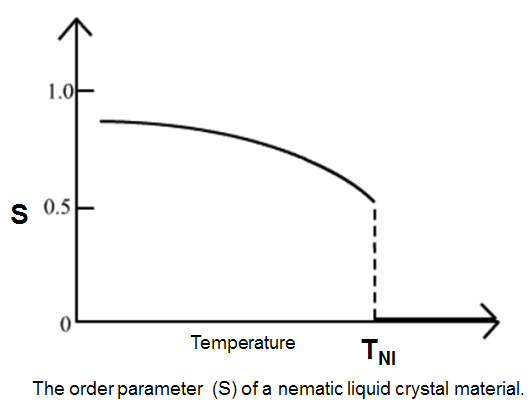
In nematic LCs the alignment isn’t perfect, though; because the molecules are in a liquid state, they keep moving around, swapping places with each other and joining other shoals.
HOW LIQUID CRYSTALS BEHAVE?
The tendency of the liquid crystal molecules to point along the director leads to a condition known as anisotropy. This term means that the properties of a material depend on the direction in which they are measured. For example, it is easier to cut a piece of wood along the grain than against it. Hence, a liquid crystal is a thermodynamic stable phase characterized by anisotropy of properties without the existence of a 3-dimensional crystal lattice, generally lying in the temperature range between the solid and isotropic liquid phase (substances exhibiting the same properties regardless the measurement direction), hence the term mesophase, used synonymously with liquid crystal state.
The distinguishing characteristic of the liquid crystalline state is exactly the tendency of the molecules (mesogens) to point along a common axis, called the director (the molecular direction of preferred 1D-orientation in liquid crystalline: mesophases). This is in contrast to molecules in the traditional liquid phase, which have no intrinsic order and likewise in contrast with the traditional solid phase which has a highly ordered 3D-structure.
About half of all known organic chemicals become liquid crystals when heated, with consistencies ranging from free-flowing liquids to semi-solids. Liquid Crystal materials generally have several common characteristics. Among these are a rod-like molecular structure, rigidness of the long axis, and strong dipole and/or easily polarizable substituents. A dipole is present when we have two equal electric or magnetic charges of opposite sign, separated by a small distance. In the electric case, the dipole moment is given by the product of one charge and the distance of separation. This applies to charge and current distributions as well. In the electric case, a displacement of charge distribution produces a dipole moment, as in a molecule. Hence the polar molecules give the liquid crystal a very useful property – they respond to applied electric fields. They do so by changing their direction of alignment. Thus you can get a whole shoal to point in a particular direction by applying a voltage. This turns out to be key to the technological success of liquid crystals; it´s what allows them to be integrated into electronic devices. A long, rigid, highly anisotropic structure being the main criterion for liquid crystalline behavior, it is also responsible for the unique optical properties exploited by scientists and engineers in a variety of applications.
To obtain this structure many liquid crystalline materials are based on benzene rings and organic carbon-based compounds such as 4-cyano-4´-pentylbiphenyl which is not so different than those found in linseed oil.
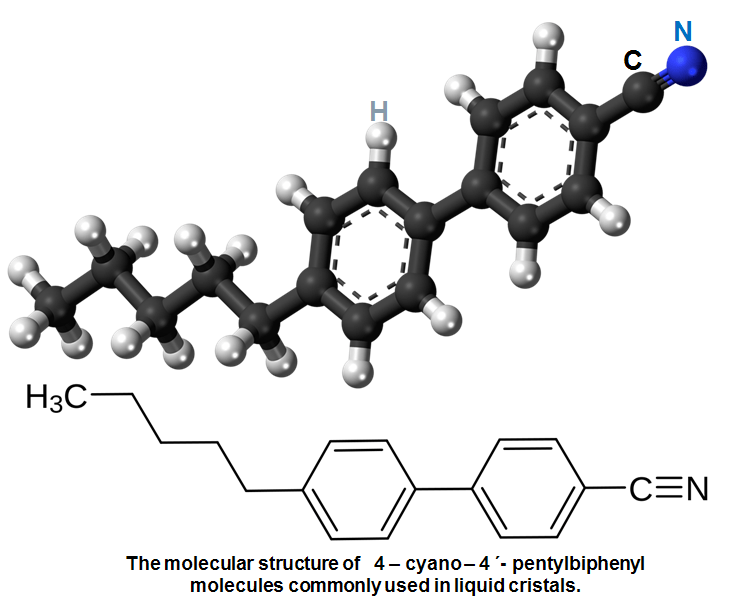
The main body of a 4-cyano-4´pentylbiphenyl molecule is made up of two hexagonal rings. This gives it its rigid structure, but the electrons binding it together are not evenly distributed: it is a polar molecule. There are areas concentrated in negative electrical charge and some concentrated in positive electrical charge. The positive charges on one molecule attract the negative charges on another, increasing the tendency of molecules to align with each other in an organized structure – a crystal. But the tail of 4-cyano-4´-pentylbiphenyl has a CH3 group on it, one that´s flexible and wriggles acting in opposition to the formation of a crystal. Hence the 4-cyano-4´-pentylbiphenyl structures are partly organized and partly fluid – it is a liquid crystal.
Above a temperature of 35°C, the influence of the CH3 tail wins and 4-cyano-4´- pentylbiphenyl behaves like a normal transparent oil. But cool it down to room temperature, and the liquid becomes milky in appearance. It´s not solid at this temperature, but something odd has happened to it. The molecules have begun to align with one other in much the same way that fish align when they´re part of a shoal. It´s very unusual for liquids to have a structure like this. One of the definitive qualities of a liquid is that its atoms and molecules have too much energy to stay in one place for any length of time, and so they are constantly rotating, vibrating and migrating. But liquid crystals are different – the molecules are still dynamic, and can flow, but they keep an alignment of their orientation, which has been compared to the regular alignment of atoms in a crystal – hence the name.
These LC materials are responsible to make moving images possible and they are the reason why we have today touchscreens and screens in general of ultra-high image quality. Liquid crystals, once a curiosity, have become important in many aspects of science, medicine, and industry as well as people’s everyday lives. It is expected that they will become even more useful in the future and that many new uses for these fascinating chemicals will become evident. Likewise because myriad organic chemicals and many chemicals of bio-organic chemical importance can be liquid crystals, the liquid crystal state is quite important.

Leave a comment