A liquid lacks both the strong spatial order of a solid, though it can have the high density of solids (a liquid has volume but doesn’t have shape) and the absence of total disorder of a gas that results from the low density of gases i.e., gas molecules are relatively free of each other’s influence (while the liquid molecules are bonded with each other). That means a liquid is the intermediate state of matter between Solid and Gas. For this reason despite the differences between liquids, they can generally be characterized by a set of common properties as we’ll see next.These properties are:
- VISCOSITY
- SURFACE TENSION
- ADHESION/COHESION
- VAPOR PRESSURE
- VOLATILITY
VISCOSITY – in simple terms viscosity is a measure that represents how easily a fluid flows.
In other words the viscosity of a fluid (liquid or gas) is a measure of its resistance to gradual deformation by shear stress or tensile stress. The shear resistance in a fluid is caused by inter-molecular friction exerted when layers of fluid attempt to slide by one another.
In a liquid, Viscosity varies according to the molecular size and intermolecular forces. Therefore, viscosity is determined by bonds between the liquid’s molecules – the stronger the bonds, the more viscous the liquid. Increasing the temperature of a liquid decreases its viscosity, because the molecules have more energy to overcome the intermolecular bonds, allowing the liquid to flow more easily. A liquid with low viscosity flows easily and is commonly said to be “thin” while a liquid with high-viscosity flows less readily therefore is said to be “thick”. For example, honey due to its larger molecular structure is more viscous than water. Honey is thicker than water and flows more slowly.

Liquid flow = Liquids with low viscosity, such as water, flow easily, because the bonds between the molecules are weak. In contrast, honey flows much less readily at the same temperature, due to the strength of its intermolecular bonds.
There are two related measures of fluid viscosity:
- dynamic (μ) (or absolute) as is the measurement of the fluid’s internal resistance to flow
- kinematic (ν) as the ratio of dynamic viscosity to density
The dynamic viscosity often measured in units as: N s/m2, Pa* s or kg/ms – where 1 Pa s = 1 N s/m2= 1 kg/(m s). Yet for common practical use a frequently used unit for it is centipoise (cP). For instance, at a temperature of 21°C and 1 atm pressure the dynamic viscosity of water is 1cP.
The kinematic viscosity is measured in m2/s or the commonly used Stoke (St) where 1 St (Stokes) = 10-4 m2/s = 1 cm2/s . In practice the common unit is centiStoke (cS). The kinematic viscosity for water at a temperature of 21 oC and 1 atm pressure is 1.0 cS
Now you might ask: What is the most viscous liquid?
The answer is: Pitch, used for road surfaces, is the most viscous liquid known. It is about 20 billion times more viscous than water at the same temperature.
SURFACE TENSION – is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet.
This term is typically used only when the liquid surface is in contact with gas (such as the air). If the surface is between 2 liquids (such as water and oil), it is called “interface tension.”
Likewise this type of force is tiny, but it’s still big enough to oppose the force of gravity on small things: this is why some insects are able to walk on the surface of ponds. If you Look carefully at a pond skater insect as it ‘walks’ on water, you”ll see that its legs are repelled by the water – this happens because the surface tension between the water and the insect’s legs generates a repulsive force that acts against gravity. Some liquid-solid interactions do the opposite and create a molecular force of attraction. This is true of water’ and glass. Take a glass of water and you will see the edges of the water are pulled up where they meet the glass. We call this the meniscus and it too is a surface tension effect.
Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Water at 20°C has a surface tension of 72.8 dynes/cm compared to 22.3 for ethyl alcohol and 465 for mercury. Surface tension is the main characteristic of liquids which is responsible for the physical phenomenon known as Wetting.
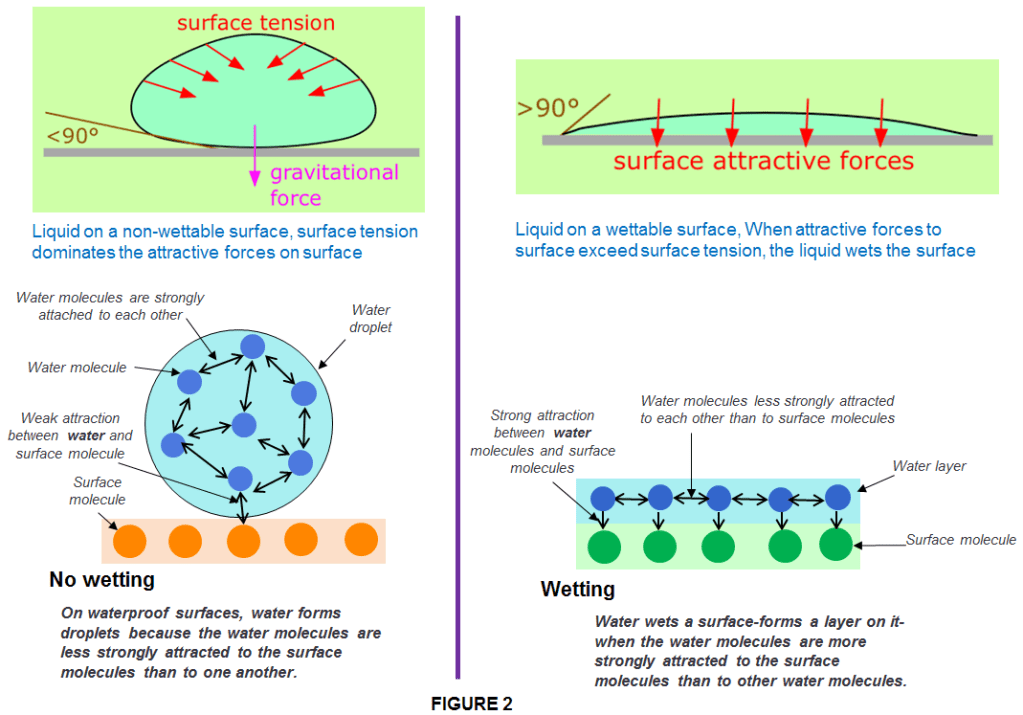
Wetting is the degree to which a liquid keeps contact with a solid surface. Whether a liquid wets a surface depends on the strength of the attractive forces within the liquid relative to the forces between the liquid and the surface.
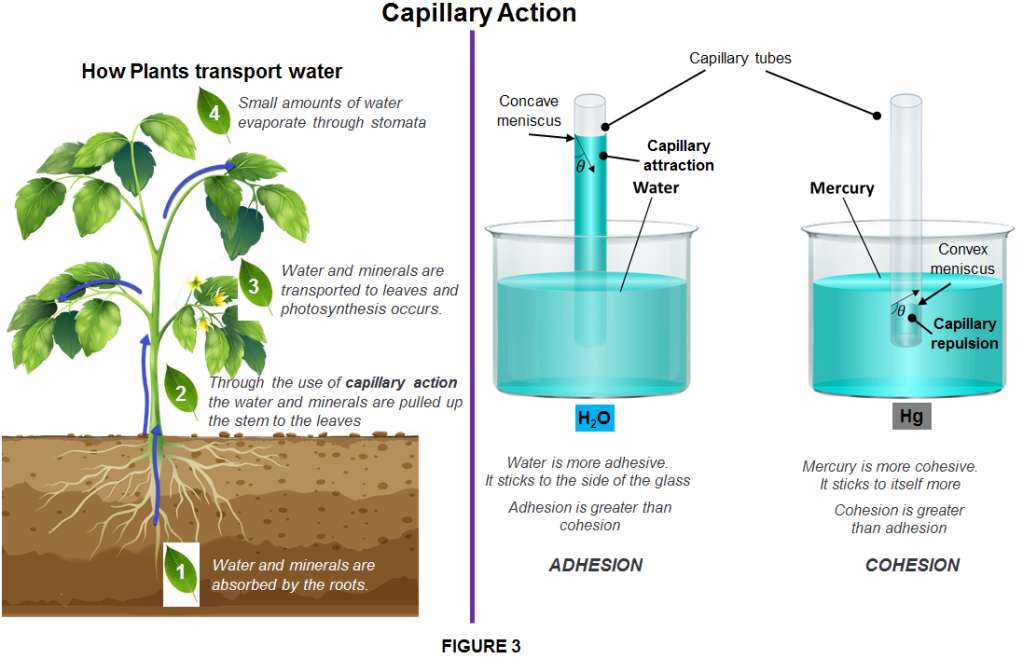
Plants have mastered this same trick. They pull water up against the force of gravity, from the ground into their bodies, using a system of tiny tubes that run through their roots, stems and leaves. As the tubes become microscopic, so the ratio of the tube’s inner surface area to the volume of liquid increases, and so the effect gets bigger. Hence manufacturers sell ‘microfibre’ cloths for window cleaning, which have microchannels similar to a plant’s. They suck up water, allowing the cloth to clean more efficiently, Kitchen tissue mops up liquid spills using the same mechanism. These are all examples of ‘wicking‘, the same surface tension effect that allows oil to climb up a string – or, more precisely, a wick.
A familiar liquid is mercury metal (Hg). Mercury is an anomaly. It is the only metal we know of that is liquid at room temperature. Mercury also has an ability to stick to itself (surface tension)—a property that all liquids exhibit. Mercury has a relatively high surface tension, which makes it very unique. If we heat liquid mercury to its boiling point of 357°C under the right pressure conditions, we would notice all particles in the liquid state go into the gas state
ADHESION/COHESION is when forces of attraction exist between different types of particles. Particles of a liquid will not only be attracted to one another, but they are generally attracted to the particles that make up the container holding the liquid, These attractive forces depend of course on the type of liquid and the other substance involved. This explains why water clings to surfaces in different ways, such as glass compared with plastic. Particles of the liquid are drawn up above the surface level of the liquid at the edges where they are in contact with the sides of the container.
When different kind of particles have the tendency to be attracted to one another we say that the liquid is adhesive; On the other way around ,when the same kind of particles have the tendency to be attracted to one another we say that the liquid is cohesive.
This happens because the molecules at the surface of a liquid do not have other like molecules on all sides of them the same like those inside the liquid have. Therefore consequently these surface molecules adhere or cohere more strongly with those associated with them on the surface. This imbalance of forces between the surface and the interior of the liquid generates a force of tension which we call surface tension as mentioned earlier.
As result this surface tension forms a surface “film” which makes it more difficult to move an object through the surface than to move it when it is completely submersed. This is what holds water together in drops (the shape with the least amount of surface area) or makes it possible to float a pin on its surface. As long as these forces of attraction are undisturbed, they can be surprisingly strong. When these liquid spheres are distorted by gravity, they form the classic raindrop shape. Water is the most cohesive nonmetallic liquid.
Adhesion also accounts for capillary action which is the tendency of a liquid to ascend narrow cylinders or permeable substances when a liquid is drawn up into such objects. One example of capillary action is when someone collects a sample of blood by touching a tiny glass tube to the blood droplet on the tip of a pricked finger. The combination of cohesive and adhesive forces means that a slight concave/convex curve, known as the meniscus, exists at the surface of most liquids. The most accurate measurement of the volume of a liquid in a graduated cylinder will be observed by looking at the volume marks closest to the bottom of this meniscus.
VAPOR PRESSURE – Pressure is the average force that a material (gas, liquid or solid) exerts upon the surface, e.g. walls of a container or other confining boundary. Vapor pressure or equilibrium vapor pressure is: the pressure that a vapor (the gaseous part of a substance) exerts on the container of said substance.
This pressure is in thermodynamic equilibrium with its condensed phases in a closed container, i.e., when conditions are such that the substance can exist in both liquid and solid state or in all three phases (solid+liquid+gas). Vapor Pressure measures the tendency of a material (solid or liquid) to change into gaseous (vapor) state.
All liquids and solids have a tendency to evaporate (in case of liquids) or sublime (in case of solids) into a gaseous form and all gases have a tendency to condense back to their liquid or solid form. When a liquid is in a confined, closed, container, an equilibrium exists between the liquid and its gaseous phase. This equilibrium exists regardless of the temperature inside the container and the temperature of the liquid. The equilibrium exists due to the fact that some of the particles in the liquid, essentially at any temperature, will always have enough energy to escape the intrinsic cohesive forces and enter the gaseous phase.
The fact that the vapor pressure is equal to the external pressure can become important when talking about boiling temperatures at various altitudes. At higher altitudes the pressure is lower than at sea level and therefore liquids such as water boil at lower temperatures. This would translate it into a longer time to cook something in the water as compared to cooking the same thing at sea level. The opposite is also true if one boils water at an altitude lower than sea level. Vapor pressures are dependent only on temperature and nothing else. The vapor pressure of a liquid does not depend on the amount of the liquid in the container, be it 1 liter or 30 liters; at the same temperature, both samples will have the same vapor pressure.
Vapor pressures have an exponential relationship with temperature and always increase as temperature increases. When a liquid is boiling, its vapor pressure is equal to the external pressure. For example, as water boils at sea level, its vapor pressure is 1 atm because the external pressure is also 1 atm. The temperature at which the vapor pressure at the surface of a liquid becomes equal to the pressure exerted by the surroundings is called the boiling point of the liquid.
Substances with high vapor pressures can form a high concentration of gas particles above the liquid in a closed system. This can be a fire hazard if the vapor is flammable. Any small spark, even one occurring from the friction between the gas particles themselves, can be enough to cause a catastrophic fire or even an explosion.
VOLATILITY can be thought of as how likely a substance will be to vaporize at normal temperatures. Volatility is more often a property of liquids, but some highly volatile solids may sublime at normal room temperature. Sublimation happens when a substance passes directly from solid to gas without passing through the liquid state. When a liquid evaporates inside a closed container, the particles cannot escape the system. Some of the evaporated particles will eventually come into contact with the remaining liquid and lose enough of their energy to condense back into the liquid. When the rate of evaporation and the rate of condensation are the same, there will be no net decrease in the amount of liquid.
Because the particles of a liquid are in constant motion, they will collide with one another, and with the sides of the container. Such collisions transfer energy from one particle to another. When enough energy (such as heat) is transferred to a particle at the surface of the liquid, it will eventually overcome the surface tension holding it to the rest of the liquid. Evaporation occurs when surface particles gain enough kinetic energy to escape the system. As the faster particles escape, the remaining particles have lower average kinetic energy, and the temperature of the liquid cools. This phenomenon is known as evaporative cooling.

Leave a comment