Antimatter is not the stuff you interact with, the same as you do with matter. It was for long time and still is a hot topic for science fiction stories. However this stuff is experimantally confirmed to be real and it definitely worth to learn more about it. So what is it?. I will try to anwer in this article.
We actually must go back right at the beginning, I mean at the moment when the Big Bang happened some 14 billion year ago. So how it all started. How and Why nobody will ever know. In the beginning there was nothing, there was only darkness on the face of the void. Then somehow a burst of energy came as if someone say “‘let there be light” and there was light, though from where it came we have no idea. This is the secret of the universe which we will never find out for sure. But what we do know is what happened next: this energy coagulated into matter and its mysterious opposite, THE ANTIMATTER, in perfect counterbalance.
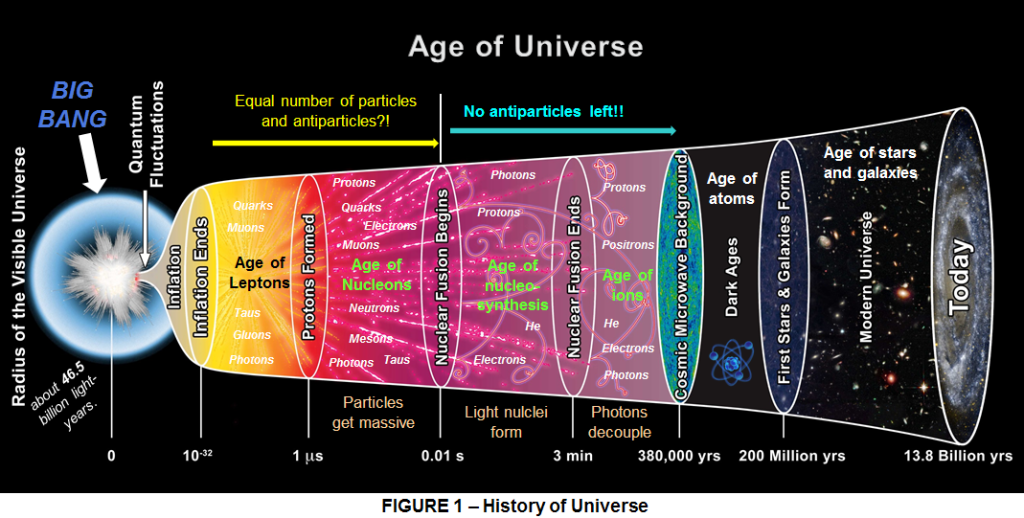
But hold on a sec, what is Antimatter? Saying that it is “the opposite of matter” is easy on the ear, but what actually is “opposite” about it? Knowing that the briefest contact with antimatter would commit whatever it touched to oblivion is awe-inspiring, what gives antimatter this power? Let’s find out.
WHAT IS ANTIMATTER?
To begin to really understand antimatter, we need first to take a voyage into ordinary matter, such as ourselves.
MATTER = Our personal characteristics are coded in our DNA, miniature helical spirals made of complex molecules. These molecules in turn are made of atoms, which are the smallest pieces of matter – such as carbon (C), hydrogen (H), iron (Fe), and so on, we have today 118 such different elements in the periodic table – that can exist and still retain the characteristics of that element. The simplest atom is hydrogen (H). It’s what the Sun is mostly made from. From all 118 element we know, Hydrogen atoms are the lightest of all and tend to float up to the top of the atmosphere and escape. For this reason hydrogen is relatively rare on earth, whereas in the universe at large it is the commonest element of all. Most of the hydrogen was made soon after the Big Bang and is nearly 14 billion years old.Vast balls of hydrogen burst into light as stars, such as our Sun. It is in the stars that the full variety of elements is fashioned. Nearly all of the atoms of oxygen (O) that you breathe, and of the carbon (C) in your skin or the ink needed to print this page, were made in stars about 5 billion years ago when the Earth was first forming.So with other words we are all stardust or, if you are less romantic, nuclear waste, for stars are nuclear furnaces with hydrogen as their primary fuel, starlight their energy output and assorted elements their ‘ash’ or waste products.
Just to have a clue, about how small atoms are: print this text and look at the dot-size at the end of the sentence. It contains some 100 billion atoms of carbon, a number far larger than all humans who have ever lived. To see any of those individual atoms with the naked eye you would need to magnify the dot to be 100 meters across. Atoms of the same element can bind is in different forms with themselves or with other elements to form a molecule. For instance water (chemically H2O) is a molecule of 2 atoms of hydrogen and 1 of oxygen, or elemental carbon (C) can bind with itself in many forms such as diamond, graphite, and carbon black-soot, charcoal, and coal.

Atoms are very small, but they are not the smallest things. It is upon entering them and encountering the basic seeds from which they are made of, that the profound duality between matter and antimatter is disclosed. Each atom contains a labyrinth of inner structure. At the centre is a dense compact nucleus, which accounts for all but a trifle (called eletron) of the atom’s mass. In other words, A hydrogen atom is made up of a positively charged proton in the middle (nucleus) and negatively charged electron cloud orbiting it. The atoms of other elements also have the same structure but their nucleus contains more than 1 proton and 1 neutron and they have shells of more than 1 electron orbiting the nucleus. While enlargement of our ink-dot to 100 meters is sufficient to see an atom, you would need to enlarge it to 10,000 kilometers, as big as the earth from pole to pole, if you wanted to see the atomic nucleus.
ANTIMATTER = When the profound entangling of space and time that comes with Einstein’s theory of relativity is married with the will-o’-the-wisp ephemeral world of uncertainty that rules within atoms, an astonishing implication emerges: it is impossible for nature to work with only the basic seeds of matter that we know. Antimatter forms chemical bonds and presumably molecules, exactly the same as matter. Antimatter is matter composed of antiparticles with the opposite electrical charge of ordinary particles and different quantum numbers.
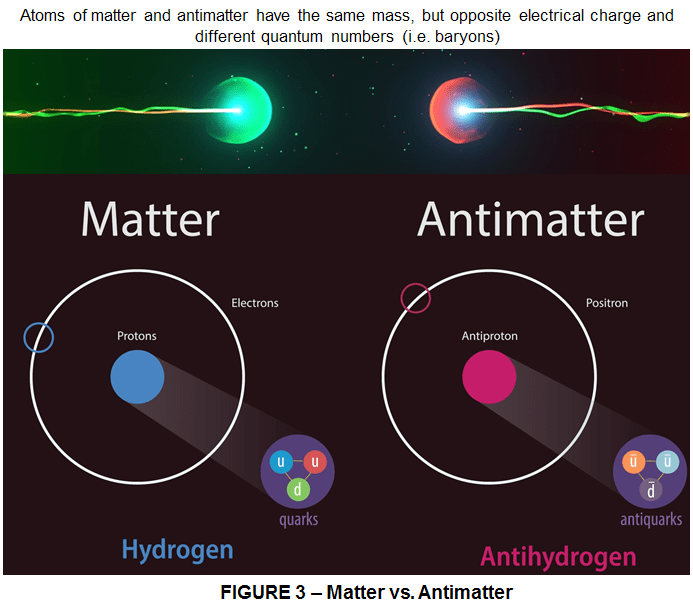
An antimatter atom (the antiatom) has a nucleus of negatively-charged antiprotons and neutral (yet different) neutrons surrounded by positively-charged antielectrons, which are called positrons. The positron has exactly the same mass as the proton. Atoms of anticarbon for instancewould make antidiamond as beautiful and hard as the diamond we know. Antisoot would be as black as soot, and the full stops in an printed version of an anti-article the same as the one you read right now. They too would need enlarging to 100 meters size for their anticarbon atoms to be seen. Were we able to do that, we would find that these smallest grains of anticarbon are indistinguishable from those of carbon. Hence what works for dots and atoms, the same is true for antidots and antiatoms. Matter and antimatter atoms and ions behave exactly the same as each other. To every variety of subatomic particle, nature is forced also to admit a negative image, a mirror opposite, each of which follows the same strict laws as do conventional particles. If suddenly everything in the universe switched from matter to antimatter, we wouldn’t know the difference. It is only when they are seen in such fine detail that the subtle choice of matter or antimatter begins to show. As the familiar particles build atoms and matter, so can these contrary versions make structures that at first sight appear to be the same as normal matter, but are fundamentally dissimilar.
MATTER VS. ANTIMATTER
Now let’s take a deeper look inside matter and see what’s going on in the atom. As I said earlier, each atom has a nucleus, within it there are protons and neutrons. In turn, the proton is a positively charged sub-atomic particle made up of 3 quacks giving it a baryon number B = 1. The baryon number is a conserved quantum number in all particle reactions. The term “conserved” means that the sum of the baryon number of all incoming particles is the same as the sum of the baryon numbers of all particles resulting from the reaction. A slight asymmetry in the laws of physics allowed baryons to be created in the Big Bang. However even at the basic level of atoms, matter and antimatter look the same: the source of their contrast is buried deeper still. The antiparticle of proton, the antiproton, has the same mass as the proton but it is negatively charges with a baryon number of B= -1 (minus one third contributed from each of the 3 antiquarks that make up the antiproton.)
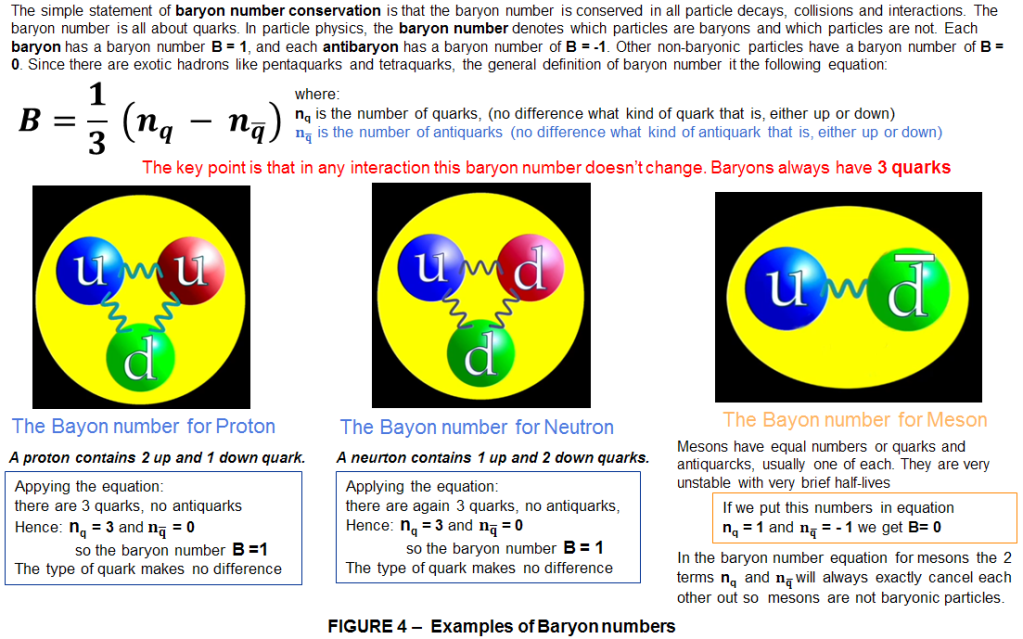
Neutral sub-atomic particles such as neutrons, are also interesting. The antineutron has the same mass and zero charge as the neutron, but it will have a baryon number of B= -1 ( again minus one third coming from each of the 3 antiquarks that make up the antineutron).
Inside atoms, besides sub-atomic particles (the electrons, and the nucleus made of protons and neutrons), we also find the 4 fundamental forces of nature:
- Gravity
- Electromagnetism (the swirling electric currents and the powerful magnetic fields)
- Strong nuclear forces
- Weak nucelar forces
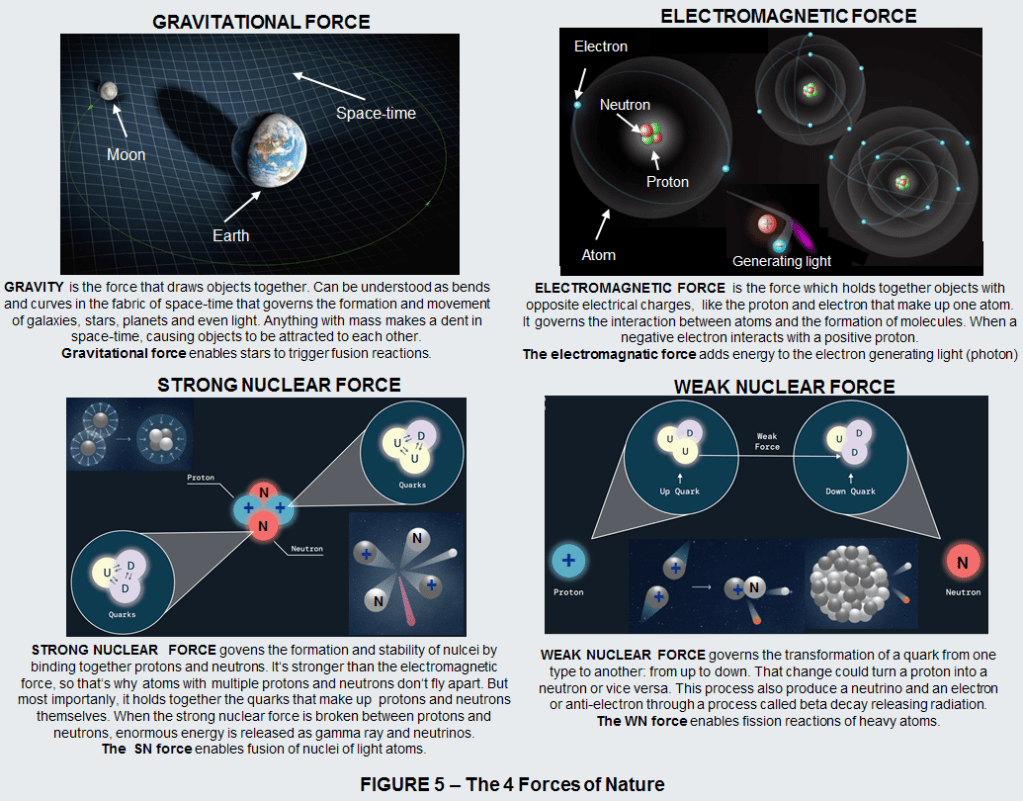
Within atoms of antimatter these currents, fields, and forces are also present, but their polarities are reversed: north poles become south; positive charges become negative.
So then , what happens when matter and antimatter interact? The answer is: fireworks!. Literally, when matter and antimatter collide, the result is: annihilation. When a positron interacts with an electron they both annihilate and the mass of the particles converts to energy, which is released as gamma photons, neutrinos, and other particles. The energy release is immense.
For example, the energy released by reacting one kilogram of matter with one kilogram of antimatter would be 1.8×1017 Joules, which is just slightly less than the yield of the largest thermonuclear weapon ever detonated, the Tsar Bomba.
But don’t worry, antimatter is scarce in the Universe nowadays. Otherwise we would have been burned by X-rays and gamma-rays every time matter and antimatter interacted.The reason that antimatter is rare in today’s Universe is that antimatter is created by very rare nuclear reactions that produce very small amount of it. The antimatter that we get from this is mostly at the sub-atomic level: positrons, antiprotons, antineutrons and antimesons.
Mesons are the proton’s and neutron’s lighter cousins that are a made up of an quark and antiquark.Yes indeed, physicists have created and studied antihydrogen, but that was under very difficult laboratory conditions that required complete isolation of the antihydrogen atoms so that they don’t interact with the walls of their container and annihilate in jet of gamma-rays. Just for context, as a matter of fact, if you were to meet your anti-self then both of you would annihilate and release the equivalent energy of roughly 2500 megatons, almost one third the total energy of the world’s arsenal of nuclear weapons!
Back to our example of the ink-dot on a printed page, imagine our ink-dot and antidot enlarged to 100 meters so that we can see their individual atoms or anti-atoms. Gently propel a tiny magnet towards the outlying regions of an atom, then launch it at an anti-atom and compare what happens. A gentle curving to the left for the one case becomes a mirror image arc to the right for the other; where once it was pulled in, now it is pushed out; where previously it was rejected, now it is sucked in; where before it was safe, now it faces annihilation. The source of these forces is the atomic nucleus, which is electrically charged. As magnets have north and south poles giving them the power to attract or repel one another, so the rule of electric charge is that like charges repel and opposite charges attract. Hence an anti-atom of antihydrogen would consist of a negative ‘antiproton’ encircled by a positive charged ‘positron’.
Paul Dirac, who first predicted in 1928 that such mirror image of matter should exist, summarized this enigma on receiving his Nobel Prize in 1933, he said, I quote:
“We must regard it rather as an accident that the Earth (and presumably the whole Solar System) contains a preponderance of negative electrons and positive protons. It is quite possible that for some of the stars it is the other way about, these stars being built up mainly of [positively charged electrons] and negative protons.”
With great prescience, and fully appreciative of the deep symmetry between the positive and negative, he commented that half of the stars could be of one kind, and half the other. These are what today we would call matter and antimatter, and as we look into the night sky at those stars, there would be no way of distinguishing them. Armed with this knowledge, we can begin to appreciate the idea of antimatter.

Leave a comment