Have no doubt that Hydrogen is a versatile energy carrier. We just have to learn more about how to harness its power. We are used to think that difficult structures are also the most difficult to control. Well… Hydrogen is just teaching us the vice-versa. Elemental Hydrogen is the simplest and lightest atom in existence (made of 1 electron and a nucleus with 1 proton), it is the most abundant substance than anything else in the universe, yet we cannot easily control it as we would like to. In spite of its ultimate atomic simplicity, Hydrogen is the most difficult substance to harness and control. Even so, there is intense activity latelly on this topic and important progess has been made. We have good reasons to be optimistic. It’s not an easy task at all, but hydrogen can be controlled. With the right technology and knowledge we can do this. We MUST do this. Giving up is not an option. Now let’s see why is so important to learn how to harness the hydrogen power. Is Hydrogen really a much better alternative to fossil fuels? Well… Absolutelly YES, it is.
We know very well that the fossil fuels we consume on daily basis are hydrocarbon fuels. And hydrocabons are nothing else but a molecular combination between carbon atoms and hydrogen atoms. So it is natural for us to compare hydrogen to other hydrocarbon fuels with which we are more familiar with, such as natural gas (methane CH4), gasoline, diesel, kerosene, ethane (C2H6); propane(C3H8), butane(C4H10), just to name the most frequently used ones. All these substances have one thing in common, they are all fossil fuels and when they burn they release more or less toxic amounts of residues in the atmosphere. Except Hydrogen.
However, in order to produce Hydrogen in a clean way is currently expensive. Likewise, hydrogen has an extremely low boiling point at -253°C, which means at standard temperature and pressue is a gas. Hydrogen is liquid only below its boiling point, which means that once produced, it needs to be stored as liquid in cryogenic conditions. If we store it as gas then we need larger storage containers and that’s already a big inconvenient. We can surely reduce the container’s size but then hydrogen gas must be kept under high-pressue condition. Yet as we very well know a fuel is much easier to store in liquid state, therefore the very low temperature of liquid Hydrogen is still a tough target to achieve. All the other hydrocarbons have higher boiling point therefore much easier to handle, besides are easier to produce. That’s one of the reason why we rely so much on fossil fuels for such a long time and for a while we still have to.
Anyway, as soon as we’ll manage produce sufficient clean hydrogen, with a reliable subsequent storage and transport infrastructure, then things will radically change, and fossil fules will really become “fossile”, I mean completelly obsolete and hence out of being commonly use. But again, we are not there yet. For now we must keep working on Hydrogen technology and until things are conveniently controlable we have no other option than to keep using fossil fuel. Meantime, let’s me show you how hydogen behaves as fuel versus fossil fuels.
CHEMICAL REACTIVITY OF HYDROGEN
All chemical fuels have a high chemical reactivity. That’s exactly one of the main reasons why we use them. This is something hydrogen does remarkably well too; in fact it does it better than any other element. In each case, a chemical reaction occurs when the fuel molecules form bonds with oxygen (from air) so that the final, reacted molecules are at a lower energy state than the initial, unreacted molecules. As the molecules react, the change in chemical energy state is accompanied by a corresponding release of energy that we can exploit to do useful work. This is true in both a combustive reaction (as in an internal combustion engine where the energy is released explosively as heat, such as in the case for hydrocarbon fuels) or in an electrochemical reaction (as in a battery or fuel cell where the energy is released as an electrical potential and heat, such as in the case of hydrogen). To put it in perspective, let’ s make the analogy with waterfalls.
The chemical energy released as result of a chemical reactivity between molecules of different substances is analogous to that which occurs when water flows from a high level to a low level. The water at the high level has potential energy that is released as kinetic energy when it falls to the low level. This kinetic energy can be harnessed to do useful work, such as turning a turbine. Once at the low level, the energy is spent and it cannot do further work at that level. In order to do further work, it must either fall to an even lower level, or be raised back to the high level through some external agency that inputs energy. The natural cycle of evaporation, condensation, and precipitation that returns water to a higher level is driven by solar and wind energy. Alternatively, a pump can return the water to a higher level, but the pump consumes a corresponding amount of energy.

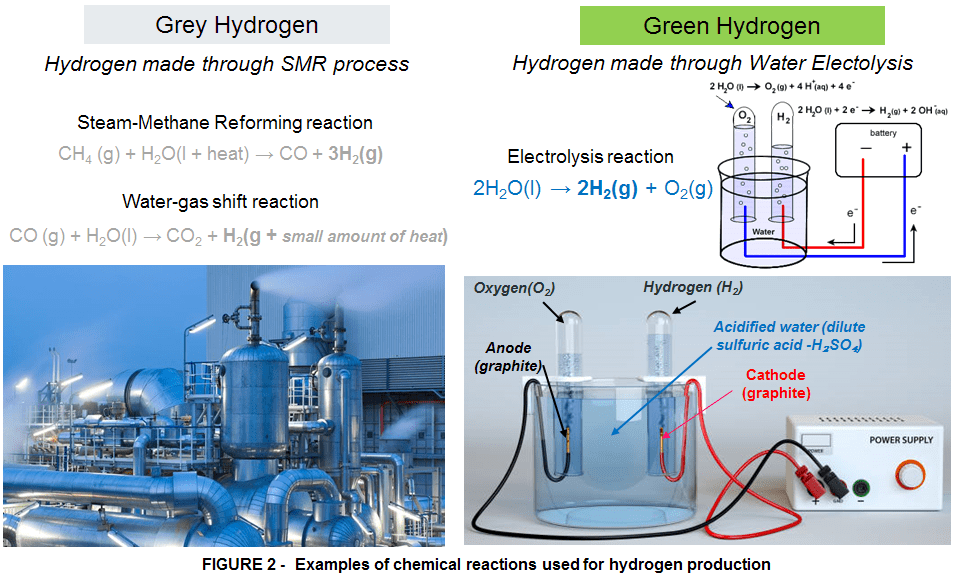
Chemical reactions of this type often require a small amount of activation energy to get started, but then the energy released by the reaction feeds further reaction in a domino effect. Thus, when a small amount of activation energy in the form of a spark is provided to a mixture of hydrogen and oxygen, the molecules react vigorously, releasing a substantial amount of heat, with water as the final product. We experience this reaction as a fire or explosion, and the resulting water vaporizes and is invisible to us since it is a superheated vapor. This water vapor can condense and become visible as it cools; this is the cloud we see when the space shuttle takes off. The water-forming reaction of hydrogen and oxygen is reversible.Thus, it is possible to convert water, at a low energy state, to hydrogen and oxygen, at a higher energy state, by adding energy slightly greater than that which was previously released (the extra to cover losses). No other type of fossil fuel is capable to do this just like hydrogen does. This is in fact the principle behind hydrogen production through water electrolysis.
CHEMICAL BY-PRODUCTS OF FUEL REACTIONS
In a chemical reaction besides the main product usually other by-products are generated as well. All fossil fuels do this. As we know from chemistry lessons in school, all of the atoms present at the start of a reaction are present at the end of the reaction although they may be reorganized into different molecules.
Hydrocarbon fuels = in addition to hydrogen and carbon, may contain other impurities such as sulfur (S). Air, in addition to being a ready source of oxygen as 21% of Earth’s atmosphere is Oxygen, also consists of 78% Nitrogen and 1% trace gases. The presence of carbon (C), nitrogen (N) and sulfur(S)(as well as unreacted hydrocarbons) result in chemical compounds during combustion that cause smog with serious health and environmental consequences. Therefore when we burn hydrocabon fuels this is what happens:
- Oxygen from the air reacts with carbon in hydrocarbons to form carbon monoxide (CO) and carbon dioxide (CO2). And as we know CO2 is benign to human beings and does not produce smog, but is a greenhouse gas and contributes to global warming. CO, on the other hand, is poisonous to humans and severely limits the blood’s ability to transport oxygen to body tissues resulting in dizziness, headaches, impaired coordination and death.The formation of CO is favored by lack of air during combustion and therefore leaner running engines emit less CO. Any reduction in CO formation is accompanied by a proportional increase in CO2 formation;
- If hydrogen is stored within then extracted from ammonia (NH3), there is nitrogen involved. Some hydrocarbon fuels might have additive containing nitrogen as well. In the presence of air, Oxygen reacts with nitrogen to form nitrogen oxides (NOx). These oxides of nitrogen damage lung tissue and act as a precursor to ozone (O3), which irritates the respiratory tract and eyes, decreases the lungs’ ability to work, and causes both cough and chest pain. The formation of NOx is favored by high combustion temperatures (1480ºC); thus, advanced ignition and increased pressure ratios tend to increase NOx emissions since these increase the combustion temperature. Lean burning engines typically reduce NOx emissions. Diesel engines, however, generate high NOx emissions when operating lean under low load conditions.
- Oxygen reacts with sulfur to form oxides of sulfur (SOx). Sulfur also forms the basis for soot, which is a form of particulate matter. Large soot particles are visible and can be filtered out of the air, or coughed out of the respiratory system. Very small soot particles (<2.5 microns) are not visible and can lodge in the lungs and cause cancer.
Hydrocarbon emissions pass into the atmosphere through incomplete combustion and evaporation. Hydrocarbons are either volatile organic compounds (VOC’s) or reactive hydrocarbons (RHC). VOC’s, such as natural gas, do not produce smog. Unburned hydrocarbons act as precursor to ozone just like NOx emissions. The RHC’s, such as gasoline, produce photochemical smog (visual pollution). The type of fuel and the use of post-combustion catalytic converters affect the amount and type of smog pollution. Light hydrocarbons are relatively rich in hydrogen and therefore provide less carbon atoms for CO and CO2 formation. Non-sulfur containing fuels eliminate SOx and soot.
=================================
Now let’s see how does this go with Hydrogen fuel.
Hydrogen = In case of Hydrogen things are a lot much better. When we burn it, Hydrogen is a nearly ideal fuel in terms of smog reduction when combusted. Hydrogen fuel contains no carbon (C) or sulfur (S), so no CO, CO2 or SOx or soot is produced during combustion (although the combustion of lubricating oil may result in trace amounts). Hydrogen allows for leaner combustion, resulting in lower combustion temperatures and very low NOx emissions. The only by-product when burning hydrogen fuel is basically water vapor.
Hydrogen is non-toxic so even the uncombusted hydrogen does not pose a direct health risk. Hydrogen is an ideal fuel in terms of smog reduction when used electrochemically in a fuel cell, rather than combusted. Hydrogen in a fuel cell produces zero harmful emissions. Oxides of nitrogen are completely eliminated due to the low operating temperature (80 ºC) of the cells. Lubricating oil is not present and is therefore not reacted. Hence, here we are, we have nearly the perfect fuel: HYDROGEN.

Leave a comment