In the last 100 years, we had so much energy available that we could simply afford the luxury of wasting it; But gradually that it is now running out and all the related environmental issues can no longer be ignored, something will have to change. And it MUST change. Our current level of energy consumption is no-longer sustainable on long term. We need better alternatives. We need a new source of energy which is abundant enough,easily accessible and as clean as possible. Well… in this case Hydrogen is definitelly a promising candidate.
In fact, We have been already putting hydrogen to use since at least the 17th century, decades before anyone really knew what it was — before Henry Cavendish first recognized it as a distinct element and named it in 1766. We just didn’t developed the necessary technology to extract and use hydrogen as fuel because crude oil is much cheaper and for a while there was available in big amounts. The technology to refine crude oil is much convenient than the technology to make clean hydrogen. Things are now starting to change.
As natural reserve on Earth, Hydrogen in elemental form is scarce. But we have a lot of Water. And water is nothing else but a molecule made of 2 hydrogen atoms and 1 atom of Oxygen, chemically known as H2O. So all we have to do is to develop an effective technology to separate the elements within the water molecule and get hydrogen out in large amounts and use it as fuel.
There are numerous ways to utilize hydrogen to enrich our lives: we can use it for heat, for light, for fertilizing crops and soon even for space travel experiences, gently lifting tourist capsules into the stratosphere., to name just a few applications. And new uses and functions of hydrogen are periodically emerging. There are still many technological drawbacks which we must overcome in the next years until we have a reliable hydrogen economy, but hydrogen has a huge potential as fuel, its benefits are highly desirable So let’s see next why hydrogen has the potential to become a vital renewable energy source of the future. The main resons in favour of HYDROGEN are:
- Is a clean fuel;
- Uses more efficient technology;
- Is convenient for heavy transport and trains;
- Has high enegy content;
- Has high energy density;
- Can be used as industrial fuel in stationary applications.
HYDROGEN IS A CLEAN FUEL
In its free state it consists of a molecule of two Hydrogen atoms (H2). When combined with oxygen (O) during its use (combustion or, more commonly, in a fuel cell), it generates only electricity, water and heat. And since in such reaction there in no carbon atom involved, no CO2 is created. These conditions are already sufficient to take on the environmental emergency, something we can no longer postpone. This means that by switching from fossil fuels to hydrogen in engines, gas turbines, boilers and fuel cells, the creation of power and heat can be done without direct CO2 emissions.
Hydrocarbons, on the other hand, are made up of carbon and hydrogen and, during combustion, when combined with oxygen, produce carbon dioxide (CO2) and other waste that is harmful to the environment and to human health (nitrogen and sulphur oxides).
HYDROGEN USES MORE EFFICIENT TECHNOLOGY
The combustion engine based on hydrocarbon fuels, launched in the middle of the 19th Century and never abandoned since, allows the car to move thanks to the combustion between fuel and air which is converted into thermal energy and in turn into mechanical energy. In almost 200 years this engine has reached its maximum performance and optimization and is today no longer sustainable due to the strong environmental impact of the waste produced.
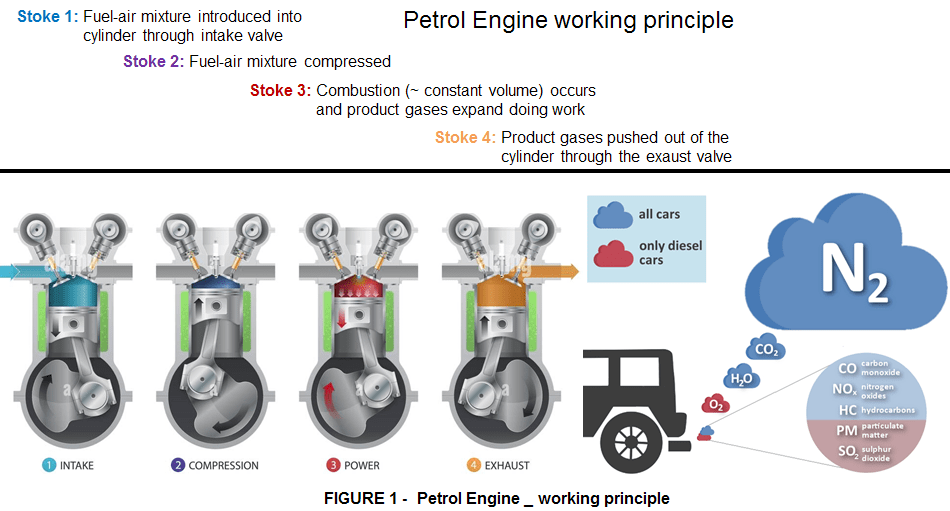
The petrol engine uses only 20/25 % of the energy introduced and consequently 75/80% of the fuel is dispersed, producing heat. This is why, you cannot touch an engine without getting burnt. Of course these figures are not strictly defined, but they are a good enough to indicate how inefficient the combustion engine powered by petrol or diesel is.
In contrast, the hydrogen uses technology which stands out for the absence of any harmful emissions. Its main use as fuel, however, is not in the combustion engine but in a fuel cell.
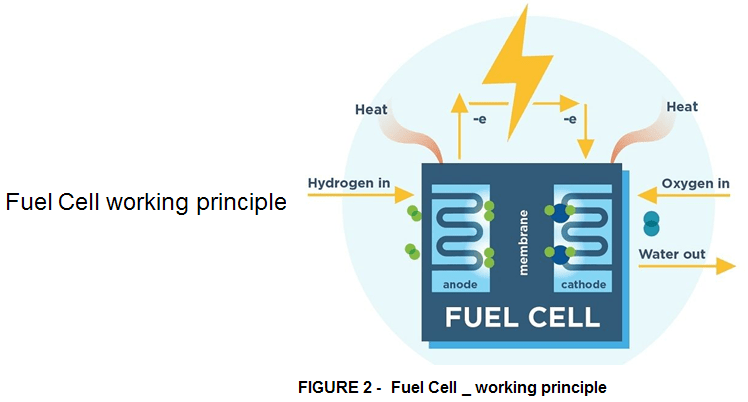
For the electric engine, the earlier mentioned percentages are entirely reversed. 80% corresponds to the energy used and only 20% corresponds to the energy dispersed. However, hydrogen is not immediately and directly exploited within a car engine because it must first be converted into electrical energy to power the engine. This passage consumes 50% of the energy and so this 80% is halved, reducing the amount of energy used to 40% which is, however, twice as much as that of a petrol engine. With the studies and experiments already under way, it is assumed that this percentage can be significantly increased, while that of the petrol/diesel engine can no longer be further optimized.
The FUEL CELL was developed for space exploration since the 1960s, whereby an electrochemical process combines hydrogen and oxygen to generate electrical energy, which in turn powers an efficient electric engine. Up to now in 2024 we’ve developed this new technology and hence hydrogen fuel-cell powered cars are available and are 100% Eco Friendly. Such cars don’t emit greenhouse gases from the tailpipe, hence they can significantly reduce the pollution in urban areas with poor air circulation such as Southern California in US and many other densely populated areas in Europe, India and China.
HYDROGEN IS A CONVENIENT FUEL FOR HEAVY TRANSPORT AND TRAINS.
Hydrogen propulsion is not yet widespread today, but one of the sectors for which it could be convenient from the outset is heavy-duty transport and trains. The goods transportation sector has been asked to bear the brunt of the responsibility for reducing greenhouse gases even though it contributes only one-third. These are means of transport which, if they were to be supplied with electric batteries as an alternative to the combustion engine, would require enormous, heavy batteries with extremely long charging times. On the contrary, hydrogen offers the advantages of a more compact propulsion system, with rapid refuelling times and a long travel range, which can be powered at charging docks located along the motorways most travelled by HGV (Heavy Goods Vehicle) fleets, without the creation of a capillary distribution network, or along the railway lines at all the main stations.
In South Korea for instance, some industrial vehicle manufacturers offer a “turnkey service” by providing HGVs for goods haulage and guaranteeing the distribution network of green hydrogen. It is easier for heavy goods vehicle manufacturers to do this than for car manufacturers because the HGVs travel along standardised routes and the rail haulage is organized in a very similar way.
To date in 2024, almost 50% of railway lines in Europe are not electrified. There are many routes in Europe where an aerial power line is just impossible, so the trains are powered by diesel, a highly polluting fuel. Hydrogen would solve both the problem of lack of electricity and the emission of pollutants. For example In Val Camonica area (in north Italy), the 104 km-long Brescia-Iseo-Edolo railway line will be served by hydrogen trains from the beginning of 2024, while Germany has had a zero-impact hydrogen line since 2018.
HYDROGEN HAS HIGH ENEGRY CONTENT
Every fuel can liberate a fixed amount of energy when it reacts completely with oxygen to form water. This energy content is measured experimentally and is quantified by a fuel’s Higher Heating Value (HHV) and Lower Heating Value (LHV). The difference between the HHV and the LHV is the “heat of vaporization” and represents the amount of energy required to vaporize a liquid fuel into a gaseous fuel, as well as the energy used to convert water to steam. The higher and lower heating values of comparative fuels are indicated in Table 1.

Hydrogen combusts quickly at high temperature. As our knowledge of how to safely handle the flammability of hydrogen has developed, this has unlocked hydrogen’s potential as a resource for our long-term energy needs, as cleaner fuels can now be made with hydrogen that are highly efficient. Although the terms HHV and LHV do not apply to batteries, just to have an idea, the energy density of a lead acid battery is approximately 0.108 kJ/g.
Liquid fuels are less efficient than gaseous fuels, some don’t even burn. Extra energy is requred in order to convert these liquid fuels in a vaporized state making them burn in a combustion process. Instead, gaseous fuels are already vaporized so no energy is required to convert them to a gas.
In case of Hydrogen gas, the water that results from both a combustive reaction and the electrochemical reaction within a fuel cell occurs as steam. Hence the lower heating value represents the amount of energy available to do external work. Both the higher and lower heating values denote the amount of energy (usually) in Joules for a given weight of fuel in kilograms. Hydrogen has the highest energy-to-weight ratio of any fuel since hydrogen is the lightest element and has no heavy carbon atoms. It is for this reason that hydrogen has been used extensively in the space program where weight is crucial.
Specifically, the amount of energy liberated during the reaction of hydrogen, on a mass basis, is about 2.5 times the heat of combustion of common hydrocarbon fuels (gasoline, diesel, methane, propane, etc.). Therefore, for a given load duty, the mass of hydrogen required is only about a third of the mass of hydrocarbon fuel needed. The high energy content of hydrogen also implies that the energy of a hydrogen gas explosion is about 2.5 times that of common hydrocarbon fuels. Thus, on an equal mass basis, hydrogen gas explosions are more destructive and carry further. However, the duration of a conflagration tends to be inversely proportional to the combustive energy, so that hydrogen fires subside much more quickly than hydrocarbon fires.
HYDROGEN HAS HIGH ENEGRY DENSITY
Whereas the energy content denotes the amount of energy for a given weight of fuel, the energy density denotes the amount of energy in Joules for a given volume in cubic meter (m3) of fuel. Thus, energy density is the product of the energy content (LHV in our case) and the density of a given fuel. The energy density is really a measure of how compactly hydrogen atoms are packed in a fuel.
It follows that hydrocarbons of increasing complexity (with more and more hydrogen atoms per molecule) have increasing energy density. At the same time, hydrocarbons of increasing complexity have more and more carbon atoms in each molecule so that these fuels are heavier and heavier in absolute terms. On this basis, hydrogen’s energy density is poor (since it has such low density) although its energy to weight ratio is the best of all fuels (because it is so light). The energy density of comparative fuels, based on the LHV, is indicated in Table 2. In comparision, the energy density of a lead acid battery is approximately 324,000 kJ/m3.

The energy density of a fuel is also affected by whether the fuel is stored as a liquid or as a gas, and if a gas, at what pressure. To put it into perspective, we can take the following 2 examples:
- Example 1 = A 500-L diesel tank containing 400kg of fuel is equivalent on an energy basis to a 8000 L volume of hydrogen gas at 3600 psi (250 bar). This is a 16 times increase in volume, although the weight of the hydrogen is only 150 kg, representing a decrease in fuel weight by a factor of about 2.8.The same diesel tank is equivalent to a 2100-L tank of liquid hydrogen. This is a 4.2 times increase in volume.
- Example 2 = If hydrogen is stored as a metal hydride, every kilogram of diesel fuel is replaced by approximately 4.5 kg of metal hydride to maintain the same hydrogen/diesel energy equivalence.Thus the same 500 L diesel tank containing 400 kg of fuel would have to be replaced with a hydride tank containing 1725 kg of “fuel” mass.
HYDROGEN CAN BE USED AS INDUSTRIAL FUEL IN STATIONARY APPLICATIONS
It is well known that Heavy Industry has a challenging decarbonization journey ahead of it, accounting for nearly 40% of the world’s final energy use in 2023. So if we’re serious about tackling climate change then we need to move away from fossil fuels in non-vehicle applications too. Hydrogen can be very well used as a fuel to power energy-intensive industrial processes, such as metal processing and glass manufacturing. Hydrogen is likely to be an important tool for replacing fossil fuels in hard-to-abate industry, in many processes of which are difficult to be electrified. Initiatives are already underway – for instance the steel manufacturer ArcelorMittal is developing industrial-scale production and use of Direct Reduced Iron (DRI) made with 100% Hydrogen.
The idea of piping hydrogen into homes or businesses seems far-fetched, but it is also possible. We don’ necessarily need to build a completelly new pipeline infrastructure. The current liquid natural gas infrastructure could be modified for hydrogen. The flammability of hydrogen for sure presents safety concerns, but with the right provisions these concerns can be mitigated — electricity is dangerous, yet we’re all using that.

Leave a comment