Hydrogen is abundant, being the most common element in the universe. The sun consumes 600 million tons of it each second. On Earth at normal temperature and pressure the element Hydrogen is a gas. Because of its atomic structure of 1 proton and 1 electron H is the lightest element in existence and has an extremely high buoyancy; hence in its pure from it quickly escape the Earth’s atmosphere. So, unlike oil, large reservoirs of hydrogen are not to be found on Earth. However exactly because of its simplicity hydrogen is the most powerful atom among all the elements. It can react in complex ways, forming different types of atomic bonds, hence although hydrogen is the simplest element and most plentiful gas in the universe; it never occurs by itself, yet is always combined with other elements such as oxygen (O) and carbon (C). The particular type of chemical bond hydrogen is able to do in molecules is called Hydrogen-Bond (Fig.1). At macroscale such bonds are weak but at intermolecular level they provide the strongest attraction among any other type of chemical bonding.
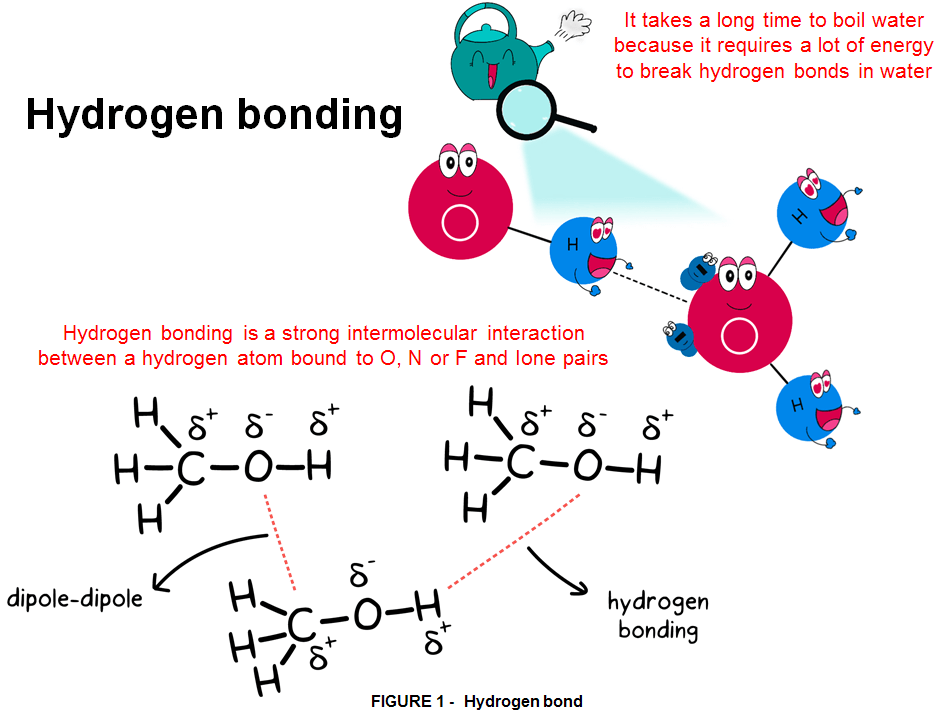
Hydrogen bonds are not as strong as covalent bonds, but they greatly influence the physical properties of many substances. However, Hydrogen bonds are a particularly strong form of dipole-dipole forces, which arise because of the unequal sharing of electrons in some covalent bonds. If one atom in a covalent bond is more electronegative than the other, it “pulls” harder on the electrons that the 2 atoms share, giving the more electronegative atom a partial negative charge, and the less electronegative atom a partial positive charge.The partially negative atom on one molecule attracts the partially positive atom on a neighboring molecule, causing the 2 molecules to be more attracted to each other than two nonpolar molecules (which have no electronegativity differences between their bonded atoms) would be. Molecules that interact by these dipole-dipole forces tend to have higher boiling points than nonpolar molecules, because higher temperatures are necessary to overcome the attractive forces between the molecules and separate the molecules into the gas phase.
In the case of O—H, N—H, and F—H bonds, the electronegativity differences are particularly large because oxygen (O), nitrogen (N) and fluorine (F) are the most strongly electronegative elements. Hence, the attractive forces between molecules containing these bonds are particularly strong. For this reason the presence of hydrogen atoms is crucial in large organic molecules, as it provides structure and stability through this special type of chemical bond. Hydrogen is therefore essential for life on Earth principally because it is crucially involved in the formation of water (the compound between hydrogen and oxygen) and organic compounds (between hydrogen and carbon) called hydrocarbons, both compounds forming exactly the basis of all living things.
THE COMPOUNDS OF HYDROGEN
Hydrogen is a highly combustible and a fairly reactive element. There are 4 main hydrogen compounds essential for life on Earth. These are:
- Water;
- Hydrocarbons;
- Acids;
- Bases.
WATER – the compound molecule between 2 atoms of Hydrogen and 1 of Oxygen, chemically identifies as H2O – is the most abundant compound available on Earth. Hydrogen bonds are primarimy found in water, and they produce a greater attraction between water molecules than would be normal. Without hydrogen bonding, water would boil and freeze at much lower temperatures.

Water is a very powerful solvent as it dissolves most substances, at least to some degree. The reason for this is because water molecules can easily separate or dissociate into H+ ions and OH ions, and these ions can bind to other ions by electrostatic attraction. In particular, hydrogen bonds are responsible for the fact that water is a liquid at temperatures at which molecules of similar molecular mass are gases. For instance, hydrogen sulfide (H2S), which weighs 34.08 g/mol, boils at -60.3°C, while water (H2O), weighing in at a measly 18.02 g/mol, boils at 100°C.
The weak bonds that hydrogen forms in molecules give water its relatively high boiling point, allowing it to exist in liquid form in the Earth’s atmosphere, while at low temperatures, the hydrogen bonds adjust and hold the oxygen atoms apart in a kind of crystal lattice: most substances are denser in their solid state than in their liquid state, but this lattice makes ice lighter than water. Ice floats on liquid water because the hydrogen bonds hold the molecules into a more open, hexagonal array, causing the solid form to be less dense than the liquid form.
HYDROCARBONS – are the organic compounds made of molecules that contain only hydrogen (H) and carbon (C) – and these include fossil fuels such as coal, crude oil and natural gas. When these fossil fuels burn, oxygen atoms combine with hydrocarbons, producing carbon dioxide (CO2) and water (H2O). This makes Hydrogen a highly combustible element. When you see a candle burning, this is mostly because hydrogen is released from the oil or tallow and burns when it comes into contact with oxygen. There are thousands of types of hydrocarbon compounds, each with a specific combination of carbon and hydrogen atoms in a unique geometry.
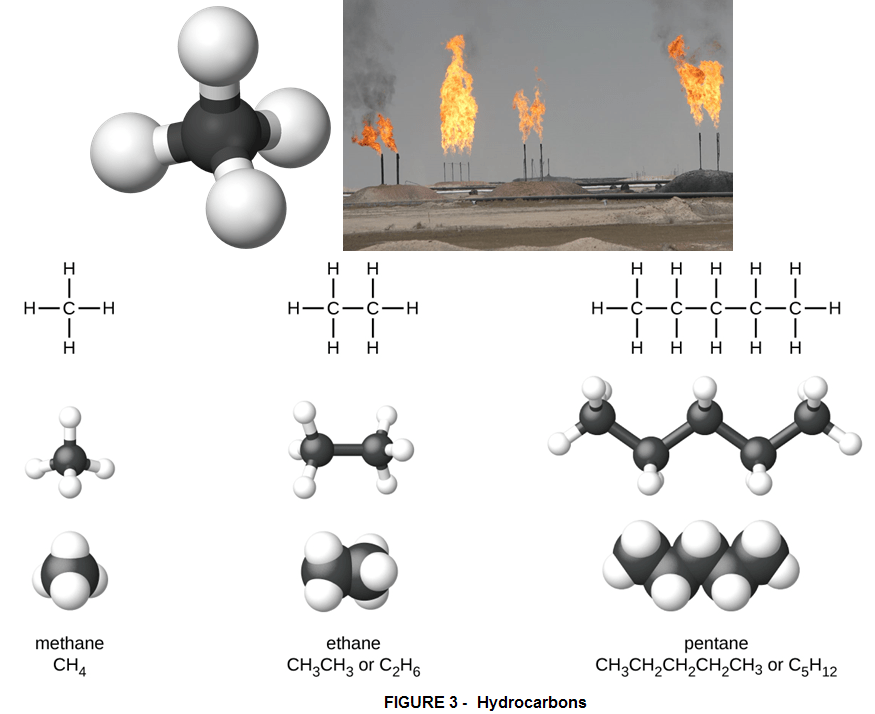
Gasoline is composed of a mixture of many different hydrocarbons, but an important constituent is heptane (C7H16). Gasoline, diesel, kerosene, and compounds found in asphalt, heavy oils and waxes, are considered heavy hydrocarbons as they contain many carbon atoms per molecule, and therefore have high molecular weight.
The lightest hydrocarbons are gases at normal atmospheric pressure and temperature. Heavier hydrocarbons, with 5 to 18 carbon atoms per compound, are liquid at ambient conditions and have increasing viscosity with molecular weight.
There are 4 main different types of hydrocarbons, which are classified as:
- ALKANES;
- ALKENES;
- ALKYNES;
- AROMATIC HYDROCARBONS.
Each of these major classes of hydrocarbons represents a family of individual hydrocarbons that share some structural feature. The classes differ in the ratio of hydrogen to carbon atoms and how the atoms are arranged.
Alkanes = also known as paraffins are the simplest type of hydrocarbon, containing single bonds of the 2 elements (Cabon and Hydrogen).
The simplest of all hydrocarbons also included here is methane (CH4), which is the principal constituent of natural gas.Other components of natural gas include ethane (C2H6), propane (C3H8), butane (C4H10) and pentane (C5H12) as well as impurities. These are all considered light hydrocarbons since they contain less than five carbon atoms per molecule and therefore have low molecular weight (a carbon atom is almost 12 times as heavy as a hydrogen atom).
It is also possible for paraffins with 4 or more carbon atoms to exist as 2 or more distinct compounds, which have the same number of carbon and hydrogen atoms. These compounds, called structural isomers, differ in the arrangement of the carbon atoms. Normal octane (n-octane) (C8H18) and iso-octane (C8H18) are two examples of 8-carbon structural isomers. Iso-octane is the common name for 2,2,4-trimethylpentane, which specifies the branching pattern of the 3 methyl groups on a pentane backbone. Like all other hydrocarbons, alkanes are excellent sources of fuel – but can be damaging to the environment when combusted.
Alkenes = contain at least one carbon atom for every carbon double bond in their chain. The double bond may be internal or in the terminal position. Terminal alkenes are also known as olefins.
Olefins (CnH2n) are similar to paraffins, but have 2 fewer hydrogen atoms and contain at least one double bond between carbon atoms. They rarely occur naturally in crude oil, but are formed during refining. Like paraffins, olefins with 4 or more carbons can exist as structural isomers. The most common and least complex alkenes are ethene (C2H4) and propene (C3H6). The double bond is what differentiates an alkene from an alkane and its exact position in the molecular chain can create different bonding structures.
Alkynes = they differ from alkanes and alkenes in that they contain a triple bond between two carbon atoms. The three most common and least complex alkynes are ethyne (C2H2), propyne (C3H4) and butyne (C4H6). All alkynes contain the suffix -yne, meaning they can be easily identified in comparison to other types of hydrocarbons
Aromatic hydrocarbons = are also true hydrocarbons containing only hydrogen and carbon. They are more complicated than the other types of hydrocarbons mentioned above and can adopt various structures. They are called aromatic due to the fact that they have a tendency to have a strong smell attached to them. Some of the carbon atoms in aromatics are arranged in a ring, but they are joined by aromatic bonds, not single bonds. Aromatic rings always contain 6 carbons; in polycyclic aromatics, like naphthalene (C10H8), some of the carbons are shared by 2 or more rings. The most common and least complex aromatic compound is benzene (C6H6). Benzene (and many other aromatic hydrocarbons) are found in fuel sources such as gasoline, diesel and kerosene.
ACIDS – are compounds with at least one hydrogen atom that can dissociate (be easily removed) to form an anion and an H+ ion (a proton) in aqueous solution, thereby forming an acidic solution. The easily-removed hydrogen atoms contained in acids, are usually connected to oxygen, nitrogen, or a halogen. When dissolved in water these substances transfer hydrogen as “H+” to water, forming the hydronium ion, H3O+. (This is a greatly oversimplified explanation of acid-base chemistry.).
BASES – are compounds that produce hydroxide ions (OH−) and a cation when dissolved in water, thus forming a basic solution.
Solutions that are neither basic nor acidic are NEUTRAL. These don’t break into ions when dissolved in water. Salts often do break into ions in water and are electrolytes, but are not necessarily acids or bases.
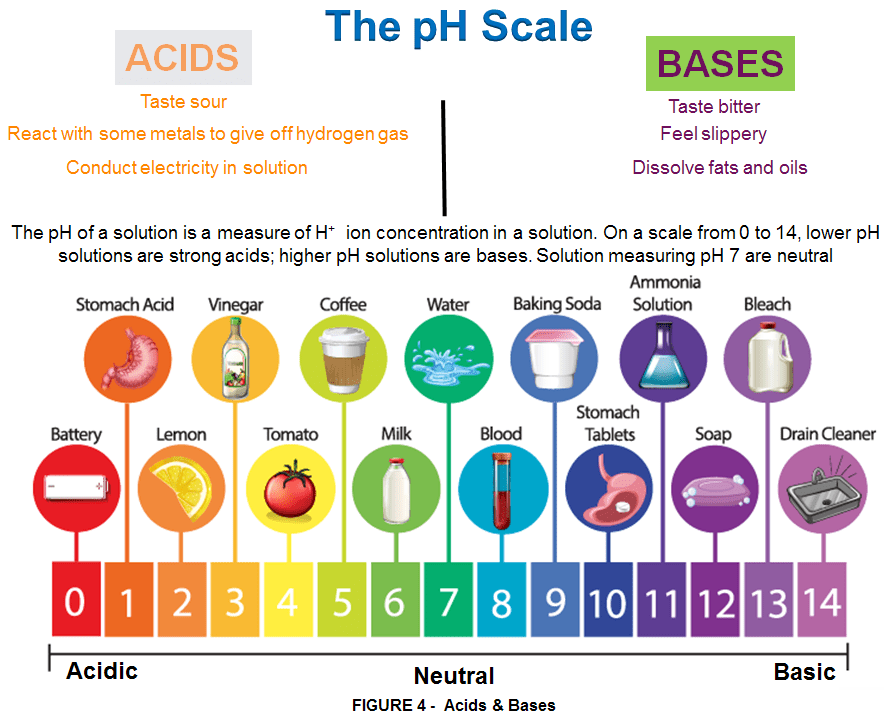
Household acids and bases are common in everyday life. Both acids and bases are electrolytes, which means they break into ions in water. Acids donate hydrogen ions (H+) or protons. Depending on the definition, bases either produce hydroxide ions (OH–), accept hydrogen ions or protons, or donate electron pairs. Acids and bases undergo a chemical reaction or neutralize each other, forming a salt and water.
=============================================
OTHER HYDROGEN COMPOUNDS – Except Oxygen and Carbon, Hydrogen can combine with nitrogen (N) (such as NH3 (ammonia) and its derivatives), sulfur (S)(hydrogen sulfide) (H2S), and the halogens (HX) where it is found in many different hydrocarbons and other organic molecules (almost all organic molecules contain at least some hydrogen atoms).
Other chemical fuels Alcohols are compounds whose molecules combine an oxygen/hydrogen atom pair (OH) with one or more hydrocarbon groups. Common alcohol fuels are methanol (CH3OH) and ethanol (C2H5OH). These may be blended with hydrocarbons for use in internal combustion engines.
Hydrogen can bond to metal atoms too, such as lithium (Li) -> Lithium hydride (LiH), calcium (Ca) -> Calcium hydride (CaH2), etc. In these compounds, the bonding is usually pictured as a metal cation combined with a hydride anion (H–). Although in general it’s diatomic, molecular hydrogen dissociates into free atoms at high temperatures. Atomic hydrogen is a powerful reductive agent, even at ambient temperature. It reacts with the oxides and chlorides of many metals, like silver (Ag), copper (Cu), lead (Pb), bismuth (Bi) and mercury (Hg), to produce free metals. Hydrogen reduces some salts to their metallic state, like nitrates, nitrites and sodium and potassium cyanide. It reacts with a number of elements, metals and non-metals, to produce hydrides, like sodium hydride (NaH), potassium hydride (KH), hydrogen sulfide (H2S) and phosphine (PH3). Atomic hydrogen produces hydrogen peroxide, H2O2, with oxygen.
Leave a comment