In science simplicity and beauty are often equated and that’s exactly the case for Hydrogen, which is the most compact and abundant element in existence. No doubt, Hydrogen is the numero uno, the ultimate The King of Elements. Its chemical symbol is the capital letter: H. Called in different languages its name is:
Hydrogenium (in latin); Hydrogen (in english); Hidrogen (in romanian) ; Hidrógeno (in spanish); Hydrogène (in french); Wasserstoff (in german), Waterstof (in dutch).
WHAT IS HYDROGEN?
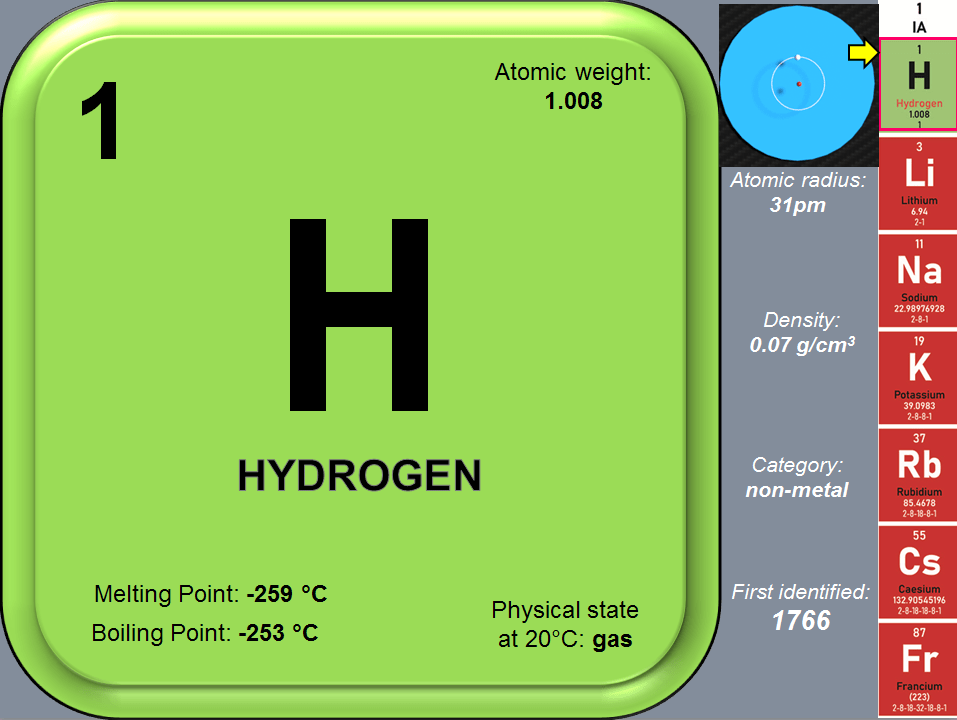
Hydrogen is the simplest, lightest and smallest possible atom in existence; it is made of only 1 electron (as particle with negative electric charge) and a nucleus of 1 proton (as particle with positive electric charge). You can visualize a hydrogen atom as a dense central nucleus with a single orbiting electron, much like a single planet in orbit around the sun. Scientists prefer to describe the electron as occupying a “probability cloud” that surrounds the nucleus somewhat like a fuzzy, spherical shell (as shown in Fig 1).
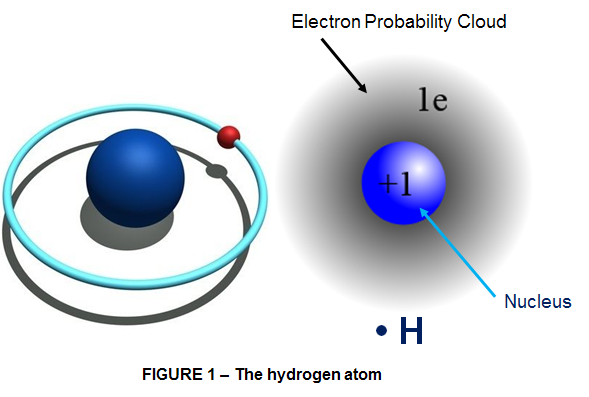
Hence hydrogen is often used as a theoretical model for more complex atoms, and the results are applied qualitatively to other atoms. Another good reason to give Hydrogen the crown as being THE ULTIMATE KING OF ELEMENTS.
Hydrogen is an essential element in the molecules involved in the processes of life, the universe and just about everything. Without hydrogen we wouldn’t have the Sun to give us heat and light. There would be no useful organic compounds to form the building blocks of life. And that most essential substance for life’s existence, water, would not exist.
TYPES OF HYDROGEN (isotopes)
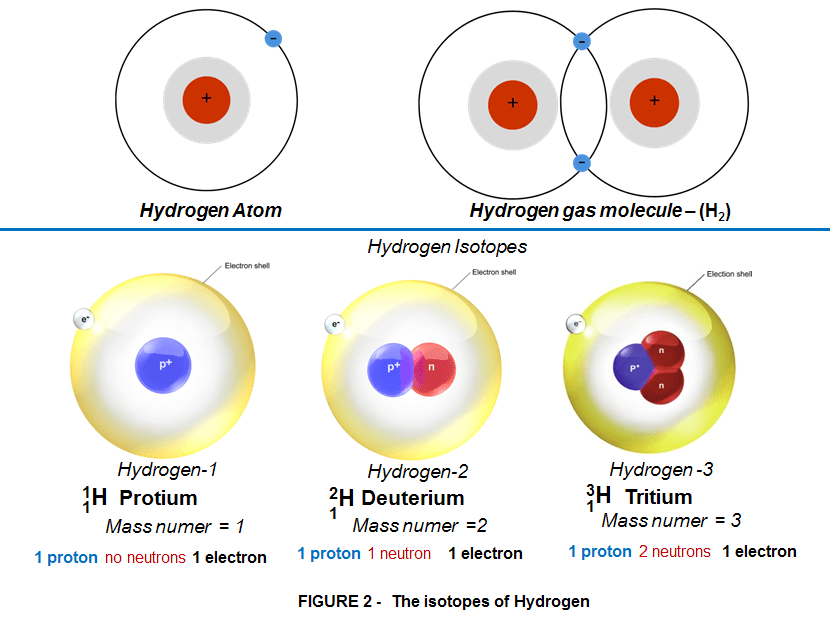
As mentioned earlier a hydrogen atom is made of 1 proton (positive charge) in its nucleus and 1 electron (negative charge) orbiting the nucleus. Yet under certain circumstances in the nucleus, besides the single proton sometimes could be also a neutron (a chargeless particle). ), the result is still a hydrogen atom but with slightly different properties.These versions of the same atom with different properties are called isotopes.
Hydrogen has 3 such versions, 2 are stable and 1 is radioactive (unstable), briefly described as follows:
- Hydrogen-1, or PROTIUM, contains 1 proton in its nucleus, and is by far the most common and stable form of hydrogen (99.985% of all the hydrogen on Earth).
- Hydrogen-2, or DEUTERIUM, contains 1 proton and 1 neutron in its nucleus, and comprises the remaining 0.015% of the world’s naturally-occurring hydrogen. It is also called “heavy hydrogen”; water made with deuterium (D2O), called heavy water, is 10% more dense than normal water. It’s quite rare but however it is a stable version of Hydrogen.
- Hydrogen-3, or TRITIUM, contains 1 proton and 2 neutrons, and is only found in trace amounts; it is produced by the interaction of cosmic rays on gases in the upper atmosphere, and in nuclear explosions, it is radioactive but since it has a half-life of only 12.3 years, it does not accumulate in the atmosphere. Although tritium can be a gas under controlled conditions, its most common form is liquid, because, like hydrogen, tritium reacts with oxygen to form water. Like ordinary water, water containing tritium, or tritiated water, is colorless and odorless. Of the three primary types of radiation, alpha, beta and gamma, tritium emits only beta radiation.
Heavy water is water made from 2 atoms of deuterium and 1 atom of oxygen- D2O. This form of water is literally heavier than “ordinary” water, since an atom of deuterium is twice as heavy as an atom of “regular” hydrogen.
- H2O has a molar mass of 18.02 g/mol;
- D2O has a molar mass of 20.03 g/mol.
Ordinary water contains about 1 molecule of D2O for every 7000 molecules of H2O. The electrolysis of water concentrates D2O in the solution, since the lighter isotope evaporates from the solution slightly faster. Successive electrolysis experiments allow pure heavy water to be produced, but it takes about 100,000 gallons of water to produce 1 gallon of heavy water by this method. (100.000 US Gallons = 378.541 Liters).
WHERE DOES HYDROGEN COMES FROM?
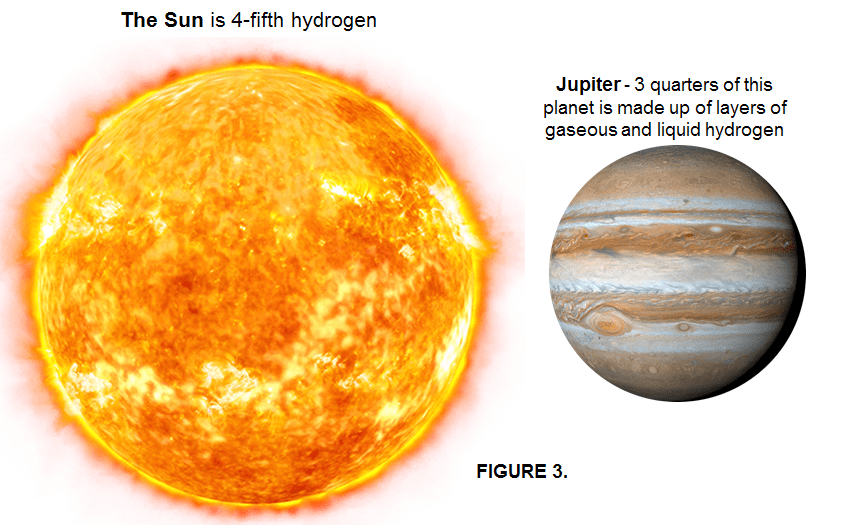
Hydrogen has been around since atoms first formed in the residue of the big bang some 13.8 billion years ago. Together with helium (He) and trace amounts of Lithium (Li), huge amount of Hydrogen (H) became concentrated into stars by the force of gravity, this making Hydrogen (H) the most abundant element in the universe by far. Despite billions of years of countless stars fusing hydrogen into helium, it still makes up 75% of the detectable content of the universe. Hydrogen (H) and helium (He) together make up 99% of the “normal” matter of the universe. (Of course, there’s also “dark matter” and “dark energy” to worry about, but that’s another story, mostly unknown). The fusion of hydrogen to form helium provides the power that makes stars shine: in the Sun, 600 million tons of hydrogen undergo fusion to form helium every second, converting 5 million tons of matter into energy featured as heat and light (This energy can be calculated by the famous Einstein equation -> E = mc2).
Likewise, the biggest planets in our solar system – such as Jupiter, Saturn, Uranus and Neptune – are vast balls of hydrogen mixed with other gases such as helium and methane.
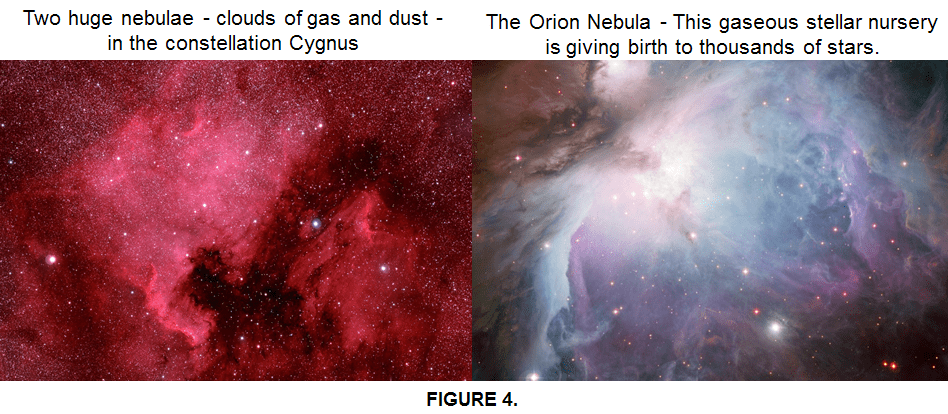
The huge clouds of dust and gas in a nebulae (as shown in Fig 4) from where stars are born are basically hydrogen. Everytime when Hydrogen is projected by the radiation from nearby stars, it produces a beautiful pinkish-red glow also known as alpha (ɑ)-Hydrogen. This effect happens because the electrons from an immense number of H atoms are raised to a high energy level and then are returned to normal, emitting photons in the process. This occurs while the huge clouds of H gas slowly collapse on themselves. The astronomers observe this “alpha hydrogen” radiation from gas clouds in every corner of the Universe.
Although hydrogen is the most abundant element in the universe (three times as abundant as helium, the next most widely occurring element), it makes up only about 0.14% of Earth’s crust by weight, therefore quite rare in its elementary form. H is found in normal air in small amounts, making up less than 1 millionth of the atmosphere. This is because being the simplest atom among all the elements – made of 1 proton and 1 electron- a Hydrogen molecule is so light that as soon found as single element it either rapidly reacts with other elements in the environment or quickly escapes the atmosphere. Hydrogen gas (H2) in its most common version found on Earth, also called dihydrogen, is made up of molecules of two hydrogen atoms.

Acidic solutions have higher concentrations of hydrogen ions (H+) than pure water. The degree of acidity of a solution, known as pH, is really a measure of the concentration of H+ ions in that solution. Acids react vigorously with most metals: metal atoms dissolve in the acid, displacing hydrogen ions and forcing them to pair up to produce hydrogen gas molecules (as shown in Fig. 6-c). Several scientists had produced hydrogen in this way long before it was known to be an element.

Likewise, Hydrogen and Carbon (C) form millions of organic compounds called hydrocarbons, which form the basis of many living things. Fossil fuels, such as oil, coal, and natural gas, are made primarily of hydrocarbons. When these fossil fuels burn, oxygen atoms combine with hydrocarbons, producing carbon dioxide (CO₂) and water (H₂O). As part of innumerable carbon compounds, hydrogen is present in petroleum and in all animal and vegetable tissue. Even though it is often said that there are more known compounds of carbon than of any other element, the fact is that, since hydrogen is contained in almost all carbon compounds and also forms a multitude of compounds with many other known elements (except some of the noble gases, for instance Neon), it is possible that hydrogen compounds are more numerous.
In addition to the hydrogen found in water molecules, living things also have hydrogen in each of their organic molecules, including proteins, carbohydrates, and fats. Hydrogen is therefore found in the Earth’s crust at a concentration of 1500 ppm, making it the 10th most abundant element on Earth.
Hydrogen is a clean, safe and versatile energy carrier. It’s never alone.
Leave a comment