If matter is exposed to an external force such as shear stress also influenced by temperature and pressure, it tends to either have a plastic deformation or starts to flow. The volume of matter which continuously changes its shape under the influence of any force or stress, it flows and the substance is called fluid.
A fluid is therefore a substance that flows under the influence of shear forces. Liquids, gases, and plasma’s are all examples of fluids. Plastic solids are also considered fluids to some extent. The shear modulus of fluid is zero, which means such substances cannot resist any force. Different substance flow at different speed is shown in Fig. 1.
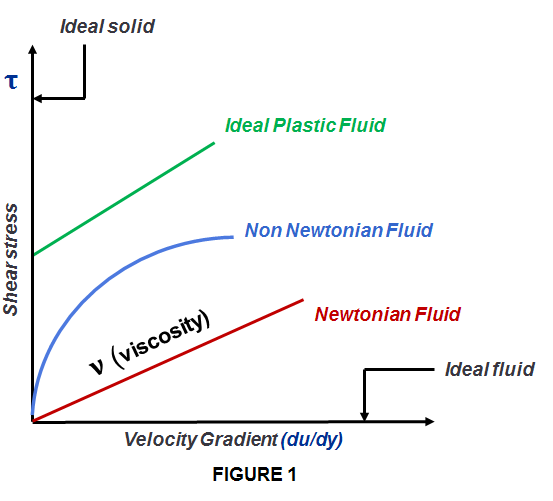
When Sir Isaac Newton used for the first time in 1687 a differential equation establishing the mathematical relationship between viscosity, shear stress and strain he discovered that for a given temperature and pressure condition the fluid matter (gas and liquid) flow with a constant viscosity regardless of external force applied, such as stirring or mixing. The fluid flow behavior remains the same. Therefore Newton established this equation of constant viscosity of fluids which we now call Newton’s Law of Viscosity and all fluids obeying Newton’s law are considered Newtonian Fluids (many liquids and most gases). Mathematically this equation is:

Yet Newton was partially right because not all fluids behave according to his law. There are cases when the fluids do not follow Newton’s law of constant viscosity; instead they have variable viscosity and variable relationship with shear stress in the same pressure and temperature condition like Newtonian fluids. These fluids are called NON-NEWTONIAN. If water or air (both being Newtonian fluids) exhibited these properties, runners and swimmers would find the surroundings thickening around them as they attempted to move faster. Therefore:
A fluid is a Non Newtonian fluid: if for a given temperature and pressure its viscosity has a variable relationship with the amount of shear rate (stress) applied (such as mixing or a sudden application of force).
Hence the viscosity of non-Newtonian fluids changes (decreases or increases), according to the type of external force applied on the fluid.
Liquids with constant viscosity at a given temperature and pressure, known as Newtonian liquids, are incompressible. This means that they exhibit a negligible change under pressure. On the other hand the Non-Newtonian liquids under the same circumstances will tend to become either thicker or thinner, they can be compressible.
Non-Newtonian fluids can also change in density when exposed to extreme temperatures, which can lead to an increase or decrease in viscosity. A fluid that has changed viscosity through one of these methods will still show a linear relationship between viscosity and shear stress. In case of liquids, with the change in viscosity under force, the substance become either more liquid or more solid. Therefore unlike Newtonian liquids, such as water, the viscosity of non-Newtonian fluids varies, depending on the force applied. For example, a cornflour and water mixture becomes thicker when a large force is applied, so a ball dropped into it from a great height will bounce off the surface while one dropped from a low height will sink.

Non Newtonian fluids are mostly liquids; however in some rare cases there are also gases that have non-newtonian behavior (such as superfluid Helium in its very rare isotope 3He).
According to their behavior under shear stress, the Non-Newtonian fluids add 4 sub-categories of viscosity to the main 2 categories (a.k.a. dynamic and kinematic viscosity as briefly introduced in my article about Newtonian Liquids). These 4 sub-categories are: Dilatant viscosity, Pseudoplastic viscosity, Rheopectic viscosity, Thixotropic viscosity.
Also important to be taken into considertion is the Bingham Plastic state which is a special case of liquids as an intermediate state between newtonian and non-newtonian state.

DILATANT FLUIDS
Dilatant fluids, also known as shear thickening fluids, are liquids or solutions whose viscosity increases as stress is applied. (see graph in Fig. 4). These fluids tend to get more viscous and thicker and flow with greater difficulty with increasing shear stress and flow. This means that (some) dilatant fluids have the unique property of being able to turn from liquid to a solid just by having stress applied. Dilatancy is not time dependent. Dilatant materials’ viscosity increases only with an increase in stress.
Solutes in dilatant solutions increase in volume when sheared. Yet when the stress is removed, the dilatant system returns to its original state of fluidity. Dilatant suspensions can be poured from a bottle, since these are reasonably fluid under these conditions.
There are not as many natural dilatants, although certain proportions of sand and water mixed together can display dilatant properties. For example at a beach, if you stand on the wet sand, your feet will sink in slightly, but if you run across the sand (thus applying greater stress to it), it will behave as a solid and your feet will not sink in.
Instead a dilatant fluid can easily artificially be made by making a 2:1 mixture like for instance of cornflour and water. It is a liquid, but when stirred it becomes thicker and more difficult to stir. If hit with a hammer, it will shatter like a brittle solid; but if left it will return to a liquid.

PSEUDOPLASTIC FLUIDS
Pseudoplastic flow exhibits the behavior of both Newtonian flow and plastic flow. In the process of pseudoplastic flow, the liquid tends to flow as plastic at high shear rates. However, it does not have a yield point, and therefore, it will always flow under the shear stress similar to a Newtonian liquid. Pseudoplastic is the opposite of dilatant; the more shear applied, the less viscous it becomes. Thus, these fluids tend to flow more easily with increasing shear stress and flow and are thus called shear-thinning fluids. Pseudoplasticity is also not time dependent. A common example of pseudoplastic behavior is blood. Wall paint is also an example of a shear-thinning fluid or pseudoplastic fluid: When applying paint to a surface, it should flow easily off the brush but not drip excessively.

A large number of pharmaceutical products, including natural and synthetic gums (e.g., liquid dispersions of tragacanth, sodium alginate, methyl cellulose, and sodium carboxymethylcellulose), exhibit pseudoplastic flow properties. Linear polymers (such as Teflon of polypropylene) in solution exhibit pseudoplastic flow.
RHEOPECTIC FLUIDS
Rheopectic is very similar to dilatant in that when shear is applied, viscosity increases. The difference here is that viscosity increase is time-dependent. These fluids are a rare class of non-Newtonian fluids. Also, they show an increased viscosity upon agitation. That means, when the fluid is shaken, it becomes thick, or it may even solidify. Moreover, higher the shear stress, more viscous that fluid becomes. It is because the microstructure of these rheopectic fluids is constructed under continuous shearing. Therefore, it is named as shear-induced crystallization. Some common examples of rheopectic fluids an example of a substance that displays rheopecty is cream; which becomes stiff only after prolonged beating.

Some other examples include gypsum pastes, printer ink, lubricants, etc.
THIXOTROPIC FLUIDS
Similar with pseudoplastic fluids, fluids with thixotropic properties decrease in viscosity when shear is applied. They are also known as “shear thinning” fluids. Yet the difference is that this type of viscosity is a time-dependent property. When exposed to a steep change in shear rate, a thixotropic fluid needs a finite amount of time to reach equilibrium viscosity. Therefore, the longer the fluid goes under shear stress, lower the viscosity of the fluid becomes.
Thixotropy is the tendency of a fluid to stay where it is. Thixotropy is created by adding specific agents to materials to help them resist flow, sag, and dripping. This property is what allows adhesives to be applied vertically, or even upside-down without moving or falling off the surface. For instance, the thixotropy of Plexus structural adhesives is what is responsible for their superior gap-filling properties in large bond lines. Thixotropy is also affected by temperature, but in a way that for some types of adhesive may be opposite to viscosity.

Some common examples of thixotropic fluids include cytoplasm of cells, synovial fluid, some varieties of honey, some types of clay, solder pastes in electronics, thread-locking fluids, gelatin, xanthan gum, etc.
BINGHAM PLASTIC
An exception to the rule is Bingham plastics, which are fluids that require a minimum stress to be applied before they flow. These are strictly non-Newtonian, but once the flow starts they behave essentially as Newtonian fluids (i.e. shear stress is linear with shear rate). It’s can be taken as 5th category different than they other 4 I mentioned above. Bingham plastics are a kind of special case of liquids as intermediate state between newtonian and non-newtonian.
Bingham plastic is a material that behaves as rigid body at low stresses but flows as a viscous fluid at high stress. This behaviour is exhibited by slurries, suspensions of solids in liquids, paints, emulsions, foams, etc. For instance toothpaste, mayonnaise, chocolate, mustard are such materials.


Leave a comment