Solid is the 1st state of matter and also the most studied and used. Anyone entering into materials science engineering the 1st stage of study starts with learning and understanding solids. In my previous article I was briefly introducing the concept of SOLID, what is it, how does it occur and what are it main properties. Let’s shortly recall the 2 main categories of structure in which this state of matter occur. These are:
CRYSTALLINE STRUCTURE: = particles are with a well-organized pattern and shape, such as a 3D structure in which all bonds between particles have equal strength. Therefore the resulted solid has a distinct melting point.
AMORPHOUS STRUCTURE:= particles that have random arrangements, so they lack an organized shape and/or pattern and melt over a range of temperatures.
That’s what happen in an ideal solid structure. In reality a solid is always more or less a mix of both. It can indeed reach a very high level of ordered pattern (crystalline) such as Diamond which is very much a crystalline structure, yet it is still not 100% crystalline. The same can be said about dis-ordered structure (amorphous) such as glass (SiO2) which is still not 100% amorphous.
Now, to go more in-depth we can have 4 other sub-categories, namely:
- COVALENT NETWORK SOLID
- IONIC SOLID
- MOLECULAR SOLID
- METALLIC SOLID
All these can exist both in crystalline and in amorphous version. However most of them frequently occur with a crystalline structure. In this article let’s focus on the COVALENT NETWORK SOLIDS.
I start with a first example. Have you ever heard of fossilized lightning? Most probably you have. When lightning strikes sand, it rapidly heats it to up to 30.000° C. That’s hotter than the Sun’s surface (which is around 5000°C°). This causes the silicon dioxide (SiO2) within the sand to turn into a crude form of glass! This glass is called sand fulgurite or “fossilized lightning” (a much cooler name). So, why does this happen?
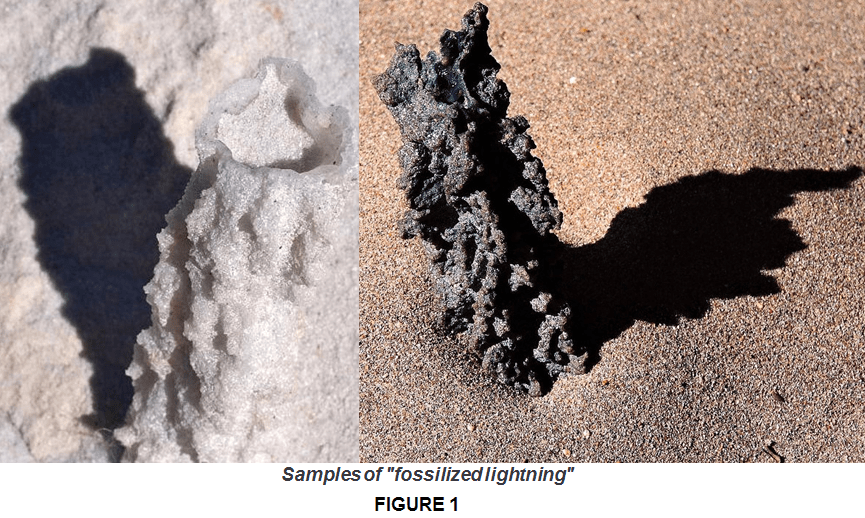
This process occurs like that because silicon dioxide is a covalent network solid, which can be either ordered (crystal) (like how it is in sand or quartz) or disordered (amorphous)(like how it is in glass).
WHAT IS A COVALENT NETWORK SOLID?
A Covalent Network Solid is = a crystal (ordered) or amorphous (non-ordered) solid in which the atoms are held together by covalent bonds in a continuous network.
A covalent bond is = a type of chemical bond where the atoms share fairly equally one or more pairs of valence electrons within the bond. This happens because the atoms are trying to fill their outermost energy level, or valence shell, with a full complement of electrons. The more equally they are shared, the more covalent character the bond has.
When the atoms share electrons in this way, they become more stable and less likely to react with other substances. This is why covalent bonds are often found in molecules, which are groups of atoms held together by covalent bonds. And because of this, there are no individually defined molecules. Hence atoms are covalently bonded in a continuous network resulting in huge crystals. Each piece of the substance is essentially one huge crystal. So the entire solid can be considered a macromolecule. By definition a unit cell is: The simplest repeating unit within a crystal.
If you think of a covalent network solid like a quilt, the unit cells are the patches that repeat across the pattern. Since the unit cell is a “patch” of the entire macromolecule, the entire “quilt” is actually this pattern repeated many times. So a continuous network of covalent bonds holds together all its constituent atoms.
In order to understand how this is possible we need to recall what the term Electronegativity is.
Electronegativity is the measurement of how much an atom wants to bond to another atom sharing electrons in a covalent bond.
The concept of electronegativity was 1st introduced by the American chemist Linus Pauling in 1932; His scale known as The Pauling Scale measures the electronegativity of an element, within a scale from 0.7 to 4. Electronegativity increases from left to right and down each column on the periodic table. The higher the value of the electronegativity, the more strongly that element attracts the shared electrons. Fluorine (F) is the most electronegative element, with an electronegativity of 3.98. while Cesium (Cs) is the least electronegative element with an electronegativity of 0.79.
Based on electronegativity there are 2 types of covalent bonding: polar and non-polar.

THE POLAR BOND = occurs between 2 or more non-metal atoms (similar or different), if the atoms have significantly different electronegativities (more than 0.4).
Polar bonds do not share electrons equally, meaning the negative charge from the electrons is not evenly distributed in the molecule. This causes a dipole moment. A dipole moment occurs when one end of the bond is positive, and the other end is negative. A classic example of a polar bond is the bond in water between hydrogen and oxygen. The valence electrons in oxygen complete hydrogen’s outer electron shell. Likewise, the valence electron in hydrogen complete oxygen’s outermost electron shell. The bond is classified as a polar bond because it has a large electronegativity difference of 1.4.
Any covalent bond between atoms of different non-metallic elements is a polar bond, but the degree of polarity varies widely and from here we can have different type of solids as well, most resulting solid are either molecular solids or ionic solids, not covalent network solids. A good example of polar bonds between the same atoms are Diamond and Graphite. Two materials with different properties but both having an atomic structure, made of a network from covalently bonded carbon atoms.
THE NON-POLAR BOND = occurs between a non-metal atom and a metalloid atom (B, Si, Ge, As, Sb, Te and Po) which share electrons equally. A bond between 2 such atoms or more atoms is non-polar if the constituent atoms have the same electronegativity or a difference in electronegativity that is less than 0.4. Like for example quartz (SiO2) (Fig3.a)
Covalent Network Solids generally occur as solids with Non-Polar bonds. A classic example is between carbon atoms resulting diamond.
Therefore in each time when atoms with an electronegativity difference of less than 2 units are joined together, the bond that is formed is a covalent bond. To be specific, the electronegativity difference between atoms in covalent bonds does not exceed 1.7. As a result of the similar electronegativity between atoms, the atoms easily share electrons.
EXAMPLES OF COVALENT NETWORK SOLIDS.
As mentioned in the example earlier, the lightning can form glass out of sand, and glasses (amorphous solids) in general are formed when the substance is rapidly heated then cooled. The atom’s initially orderly structure is disrupted, and the rapid cooling prevents atomic ordering from occurring. So the Silicon dioxide as SiO2 (as glass) is one of the most commonly known type of covalent network solid. Silicon dioxide (glass-as fused silica) is an amorphous covalent network solid. (Fig 3 b.)

In it’s crystalline form the silicon dioxide is called quartz, (Fig 3 a) which is the second most abundant material in the earth’s crust (after Oxygen). The chemical formula for quartz is SiO2, but this formula only indicates the ratio of silicon to oxygen and is not meant to imply that there are distinct SiO2 molecules present. Each silicon atom is bonded to 4 different oxygen atoms and each oxygen atom is bonded to 2 different silicon atoms creating a large network of covalent bonds.
Since quartz is symmetrical and rigid, while glass is not, it can be subjected to greater temperatures and pressures (i.e. it is stronger). Even though the formula is SiO2, you’ll see that silicon is bonded to three oxygen. And as mentioned previously, there are no individual molecules in a covalent network solid. You can’t isolate a SiO2 molecule because there aren’t any.

Another most common example of CN solid is the 3 different arrangements of carbon, namely: Diamond, Graphite and Fullerene (Fig. 4 a, b & c). While these 3 materials are made of the same very simple component – just carbon atoms – their appearance and behavior are completely different because of the different types of bonding in the solids. Graphite for instance is an exceptional example, it is a 2D network solid composed of planar sheets of covalent crystals that are held together in layers by non-covalent forces. These layers are relatively slippery and can slide past each other. For this reason, unlike typical covalent solids, graphite is very soft and electrically conductive.This ability of a single element to form solids with different crystalline arrangements is called allotropy.
Diamond is a 3D crystal and is a covalent network solid because each C atom from its structure makes 4 covalent bonds to 4 other C atoms. A diamond is essentially one huge molecule. Graphite may also be regarded as a network solid, even though there is no bonding in the z direction. Each layer, however, is an “endless” bonded network of carbon atoms. And the C60 molecule, called buckminsterfullerene, though the shorter name fullerene is often used, is a sphere composed of 6-member and 5-member carbon rings. These balls are sometimes fondly referred to as “Bucky balls”. Fullerenes are an entire class of pure carbon compounds rather than a single compound.
Covalent Network Solids include also crystals of some other nonmetals, and some covalent compounds such as silicon carbide (carborundum, the abrasive on sandpaper). Amethysts, rubies, sapphire and many other minerals have networks of covalent bonds.
PROPERTIES OF COVALENT NETWORK SOLIDS.
The properties of CN solids are largelly due to the covalent bonding within their atomic structure. Covalent network solids have:
LOW ELECTRICAL CONDUCTIBILITY = generally CN solids are poor conductors of electricity, hence are excellent electrical insulators. The reason behind is because the covalent bonds are more rigid and the angles between atoms are strictly defined versus other type of chemical bondings (ionic, molecular or hydrogen), making the energy transfer more difficult. However the conductivity of a network solid is dependent on the type of bonding.
For instance, if the molecules are composed of planar sheets of covalent crystals that are held together in layers by intermolecular forces (have delocalized electrons), like graphite or mica, these solids have high electrical conductivity. This is because electricity can “flow” across these delocalized electrons, hence making graphite a very good conductor of electricity.
On the other hand, molecules that are only covalent bonded (do not have delocalized electrons), like diamond or quartz, have low electrical conductivity. This is because all the electrons are held in place by the covalent bonds, so there is no “room” for the movement of electrons. Graphite is black because it contains an immense number of alternating double bonds, which results in a very small energy difference between the individual molecular orbitals. Thus light of virtually all wavelengths is absorbed. Diamond, on the other hand, is colorless when pure because it has no delocalized electrons.
LOW TERMAL CONDUCTIVITY = For the same reasons like for electrical conductibility, most CN solids are poor heat conductors too; although their ability to conduct heat is variable. Diamond is an exception and is one of the most thermally conductive substances known, while SiO2 (as Quartz) is about 100 times less thermally conductive.
HIGH HARDNESS = Except Mica and graphite which are soft and electrically conductive, the most covalent network solids are very hard. As exemplified by diamond, which is the hardest known substance, it can easily withstand a pressure of 6 million atmospheres. These are some extremely strong bonds. However since the amorphous CN solids are less rigid they are also softer.
Lonsdaleite as rare form of diamond, is the hardest solid known, almost 60% harder than normal diamonds.

Fine watches and, increasingly, other electronic devices use sapphire crystals instead of glass because the strong network bonding makes sapphire incredibly hard (in fact sapphire, it is the 3rd hardest substance known) and scratch-resistant.
BRITTLE = Covalent Network Solids are also known to be extremely difficult to break. This property is often associated with materials like ceramics, which are highly resistant to compression but when subjected to tensile stresses they can be very brittle and prone to cracking or fracturing without changing shape much. This is because, all the electrons are engaged in covalent bonds between atoms, thus rendering them immobile and unable to move! These materials tend to fracture with little to no permanent deformation under loading. Cracks pass easily through these materials, because the atoms cannot move to absorb the stress.
All compounds with the diamond and related structures are hard and not easily deformed, instead, they tend to shatter when subjected to large stresses. However, deformations that don’t require breaking of these bonds are easier to make, such as sliding sheets of graphite (this disrupts only the intermolecular forces, not the bonds).
Note: there is no well-defined point that classifies brittleness, but typically a material that fractures at a strain of less than 5% is considered a brittle material.
HIGH MELTING POINTS = by virtue of their strong network of covalent bonds through the sample which are very difficult to break and transform the solid into liquid, this category of solids are among the highest-melting substances known. The melting point of diamond is over 3.500°C, while the melting point of SiO2 as a type of quartz called tridymite is around 1,650°C while graphite melts at 4.489°C.
Silicon carbide (SiC) is also a CN solid with a melting point = 2986°C and used commercially as an abrasive in sandpaper and grinding wheels. It is difficult to deform or melt these and related compounds because strong covalent (C–C or Si–Si) or non-polar covalent (Si–C or Si–O) bonds must be broken, which requires a large input of energy.
INSOLUBLE = Also due to very strong covalent bonds CN solids are generally insoluble in any solvent. When species dissolve, the solute particles (dissolving species) must fit in between the solvent particles (species that does the dissolving). Because the macromolecules in CN solids are too large, this makes them difficult to dissolve. Diamond rings probably wouldn’t be as valuable if the band and the stone dissolved in the shower.

Leave a comment